Monosynaptic tracing: a step-by-step protocol
- PMID: 31408693
- PMCID: PMC11526806
- DOI: 10.1016/j.jchemneu.2019.101661
Monosynaptic tracing: a step-by-step protocol
Abstract
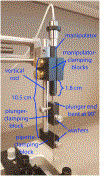
Monosynaptic tracing using deletion-mutant rabies virus allows whole-brain mapping of neurons that are directly presynaptic to a targeted population of neurons. The most common and robust way of implementing it is to use Cre mouse lines in combination with Cre-dependent adeno-associated viral vectors for expression of the required genes in the targeted neurons before subsequent injection of the rabies virus. Here we present a step-by-step protocol for performing such experiments using first-generation (ΔG) rabies viral vectors.
Copyright © 2019. Published by Elsevier B.V.
Figures

References
-
- Ahrlund-Richter S, Xuan Y, van Lunteren JA, Kim H, Ortiz C, Pollak Dorocic I, Meletis K, Carlen M, 2019. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat. Neurosci 22, 657–668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

