Roles of Myosin-Mediated Membrane Trafficking in TGF-β Signaling
- PMID: 31408934
- PMCID: PMC6719161
- DOI: 10.3390/ijms20163913
Roles of Myosin-Mediated Membrane Trafficking in TGF-β Signaling
Abstract
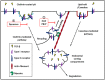
Recent findings have revealed the role of membrane traffic in the signaling of transforming growth factor-β (TGF-β). These findings originate from the pivotal function of TGF-β in development, cell proliferation, tumor metastasis, and many other processes essential in malignancy. Actin and unconventional myosin have crucial roles in subcellular trafficking of receptors; research has also revealed a growing number of unconventional myosins that have crucial roles in TGF-β signaling. Unconventional myosins modulate the spatial organization of endocytic trafficking and tether membranes or transport them along the actin cytoskeletons. Current models do not fully explain how membrane traffic forms a bridge between TGF-β and the downstream effectors that produce its functional responsiveness, such as cell migration. In this review, we present a brief overview of the current knowledge of the TGF-β signaling pathway and the molecular components that comprise the core pathway as follows: ligands, receptors, and Smad mediators. Second, we highlight key role(s) of myosin motor-mediated protein trafficking and membrane domain segregation in the modulation of the TGF-β signaling pathway. Finally, we review future challenges and provide future prospects in this field.
Keywords: TGF-β; clathrin-coated pits; endocytosis; lipid-rafts; subcellular trafficking; unconventional myosin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

