Corrosion and Oxidation Behavior of a Fe-Al-Mn-C Duplex Alloy
- PMID: 31408985
- PMCID: PMC6720586
- DOI: 10.3390/ma12162572
Corrosion and Oxidation Behavior of a Fe-Al-Mn-C Duplex Alloy
Abstract
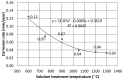
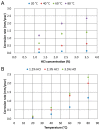
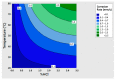
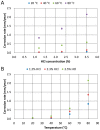
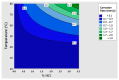
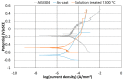
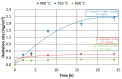
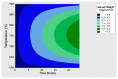
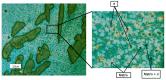
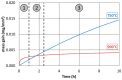
The low-density steels represent a topic of great interest within the scientific world because of the great demand from the steel market of increasingly lighter materials, also featured by an optimal mix of the mechanical properties. In this work, the corrosion and hot oxidation resistance of a Fe-15%Mn-9.5%Al-6.5%Ni-1%Cr-0.43%C were analyzed and related to the microstructural features. The material behavior was analyzed both in the as-cast and in the heat-treated state. For the corrosion test, the experimental plan was fulfilled using four different concentrations of HCl and four temperatures. In the case of hot oxidation resistance, the exposure time and the temperature effects were evaluated. The corrosion resistance in HCl was comparable to the stainless steel, and the iso-corrosion curves showed excellent resistance of the 1300 °C solution-treated material, especially at low temperatures, but it is also good at high temperatures due to the hot oxidation.
Keywords: Fe-Mn-Al-Ni steel; corrosion; light steel; potentiodynamic curves.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Frommeyer G., Brüx U. Microstructures and mechanical properties of high-strength Fe-Mn-Al-C light-weight TRIPLEX steels. Steel Res. Int. 2006;77:627. doi: 10.1002/srin.200606440. - DOI
-
- Zhang L., Song R., Zhao C., Yang F., Xu Y., Peng S. Evolution of the microstructure and mechanical properties of an austenite-ferrite Fe-Mn-Al-C steel. Mater. Sci. Eng. A. 2015;643:183–193. doi: 10.1016/j.msea.2015.07.043. - DOI
-
- Wu Z.Q., Ding H., An X.H., Han D., Liao X.Z. Influence of Al content on the strain-hardening behavior of aged low density Fe-Mn-Al-C steels with high Al content. Mater. Sci. Eng. A. 2015;639:187–191. doi: 10.1016/j.msea.2015.05.002. - DOI
-
- Lee S., Jeong J., Lee Y.-K. Precipitation and dissolution behavior of κ-carbide during continuous heating in Fe-9.3Mn-5.6Al-0.16C lightweight steel. J. Alloy. Compd. 2015;648:149–153. doi: 10.1016/j.jallcom.2015.06.048. - DOI
-
- Yang F., Song R., Li Y., Sun T., Wang K. Tensile deformation of low density duplex Fe-Mn-Al-C steel. Mater. Des. 2015;76:32–39. doi: 10.1016/j.matdes.2015.03.043. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

