Anti-Angiogenic Effect of Asperchalasine A Via Attenuation of VEGF Signaling
- PMID: 31408989
- PMCID: PMC6722956
- DOI: 10.3390/biom9080358
Anti-Angiogenic Effect of Asperchalasine A Via Attenuation of VEGF Signaling
Abstract
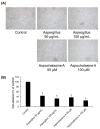
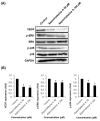
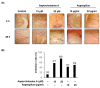
Cytochalasans are a group of structurally diverse fungal polyketide-amino acid hybrid metabolites that exhibit diverse biological functions. Asperchalasine A was identified and isolated from an extract of the marine-derived fungus, Aspergillus. Asperchalasine A is a cytochalasan dimer which consists of two cytochalasan molecules connected by an epicoccine. This study investigated the potential antiangiogenic effects of Aspergillus extract and asperchalasine A, which significantly inhibited cell adhesion and tube formation in human umbilical vein endothelial cells (HUVECs). Aspergillus extract and asperchalasine A decreased the vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR)-2 mRNA expression in a concentration-dependent manner. In addition, Aspergillus extract and asperchalasine A inhibited angiogenesis via downregulation of VEGF, p-p38, p-extracellular signal-regulated protein kinase (ERK), p-VEGFR-2, and p-Akt signaling pathways. Moreover, Aspergillus extract and asperchalasine A significantly inhibited the amount of blood vessel formation in fertilized chicken eggs using a chorioallantoic membrane assay. Our results provide experimental evidence of this novel biological activity of the potential antiangiogenic substances, Aspergillus extract, and asperchalasine A. This study also suggests that Aspergillus extract and its active component asperchalasine A are excellent candidates as adjuvant therapeutic substances for cancer prevention and treatment.
Keywords: HUVEC; VEGF; angiogenesis; asperchalasine A; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Folkman J., Cotran R. Relation of vascular proliferation to tumor growth. Int. Rev. Exp. Pathol. 1976;16:207–248. - PubMed
-
- Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C.O., Buerk D.G., Huang P.L., Jain R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

