Transient Heat Waves May Affect the Photosynthetic Capacity of Susceptible Wheat Genotypes Due to Insufficient Photosystem I Photoprotection
- PMID: 31408991
- PMCID: PMC6724146
- DOI: 10.3390/plants8080282
Transient Heat Waves May Affect the Photosynthetic Capacity of Susceptible Wheat Genotypes Due to Insufficient Photosystem I Photoprotection
Abstract
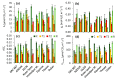
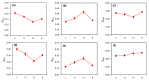
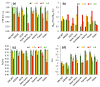
We assessed the photosynthetic responses of eight wheat varieties in conditions of a simulated heat wave in a transparent plastic tunnel for one week. We found that high temperatures (up to 38 °C at midday and above 20 °C at night) had a negative effect on the photosynthetic functions of the plants and provided differentiation of genotypes through sensitivity to heat. Measurements of gas exchange showed that the simulated heat wave led to a 40% decrease in photosynthetic activity on average in comparison to the control, with an unequal recovery of individual genotypes after a release from stress. Our results indicate that the ability to recover after heat stress was associated with an efficient regulation of linear electron transport and the prevention of over-reduction in the acceptor side of photosystem I.
Keywords: heat stress; high temperatures; photoinhibition; photoprotection; photosynthesis; photosystem I; wheat.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Pachauri R.K., Meyer L.A., editors. Climate Change 2014: Synthesis Report. IPCC; Geneva, Switzerland: 2014. Intergovernmental Panel on Climate Change.
-
- Nuttall J.G., Barlow K.M., Delahunty A.J., Christy B.P., O’Leary G.J. Acute high temperature response in wheat. Agron. J. 2018;110:1296–1308. doi: 10.2134/agronj2017.07.0392. - DOI
-
- FAOSTAT Production of Wheat in World. [(accessed on 23 February 2019)]; Available online: http://www.fao.org/faostat/en/#data/QC/visualize.
-
- Slafer G.A., Savin R., Sadras V.O. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014;157:71–83. doi: 10.1016/j.fcr.2013.12.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

