Impact of Astrocyte Depletion upon Inflammation and Demyelination in a Murine Animal Model of Multiple Sclerosis
- PMID: 31409036
- PMCID: PMC6719128
- DOI: 10.3390/ijms20163922
Impact of Astrocyte Depletion upon Inflammation and Demyelination in a Murine Animal Model of Multiple Sclerosis
Abstract
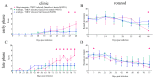
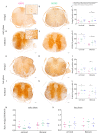
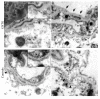
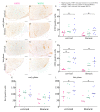
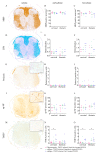
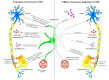
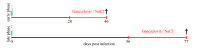
Astrocytes play a key role in demyelinating diseases, like multiple sclerosis (MS), although many of their functions remain unknown. The aim of this study was to investigate the impact of astrocyte depletion upon de- and remyelination, inflammation, axonal damage, and virus distribution in Theiler`s murine encephalomyelitis (TME). Groups of two to six glial fibrillary acidic protein (GFAP)-thymidine-kinase transgenic SJL mice and SJL wildtype mice were infected with TME virus (TMEV) or mock (vehicle only). Astrocyte depletion was induced by the intraperitoneal administration of ganciclovir during the early and late phase of TME. The animals were clinically investigated while using a scoring system and a rotarod performance test. Necropsies were performed at 46 and 77 days post infection. Cervical and thoracic spinal cord segments were investigated using hematoxylin and eosin (H&E), luxol fast blue-cresyl violet (LFB), immunohistochemistry targeting Amigo2, aquaporin 4, CD3, CD34, GFAP, ionized calcium-binding adapter molecule 1 (Iba1), myelin basic protein (MBP), non-phosphorylated neurofilaments (np-NF), periaxin, S100A10, TMEV, and immunoelectron microscopy. The astrocyte depleted mice showed a deterioration of clinical signs, a downregulation and disorganization of aquaporin 4 in perivascular astrocytes accompanied by vascular leakage. Furthermore, astrocyte depleted mice showed reduced inflammation and lower numbers of TMEV positive cells in the spinal cord. The present study indicates that astrocyte depletion in virus triggered CNS diseases contributes to a deterioration of clinical signs that are mediated by a dysfunction of perivascular astrocytes.
Keywords: GFAP-thymidine kinase transgenic SJL mice; Theiler`s murine encephalomyelitis; aquaporin 4; astrocytes; blood-spinal cord barrier; glial fibrillary acidic protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

