The effect of ICU-tailored drug-drug interaction alerts on medication prescribing and monitoring: protocol for a cluster randomized stepped-wedge trial
- PMID: 31409338
- PMCID: PMC6692933
- DOI: 10.1186/s12911-019-0888-7
The effect of ICU-tailored drug-drug interaction alerts on medication prescribing and monitoring: protocol for a cluster randomized stepped-wedge trial
Abstract
Background: Drug-drug interactions (DDIs) can cause patient harm. Between 46 and 90% of patients admitted to the Intensive Care Unit (ICU) are exposed to potential DDIs (pDDIs). This rate is twice as high as patients on general wards. Clinical decision support systems (CDSSs) have shown their potential to prevent pDDIs. However, the literature shows that there is considerable room for improvement of CDSSs, in particular by increasing the clinical relevance of the pDDI alerts they generate and thereby reducing alert fatigue. However, consensus on which pDDIs are clinically relevant in the ICU setting is lacking. The primary aim of this study is to evaluate the effect of alerts based on only clinically relevant interactions for the ICU setting on the prevention of pDDIs among Dutch ICUs.
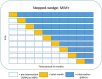
Methods: To define the clinically relevant pDDIs, we will follow a rigorous two-step Delphi procedure in which a national expert panel will assess which pDDIs are perceived clinically relevant for the Dutch ICU setting. The intervention is the CDSS that generates alerts based on the clinically relevant pDDIs. The intervention will be evaluated in a stepped-wedge trial. A total of 12 Dutch adult ICUs using the same patient data management system, in which the CDSS will operate, were invited to participate in the trial. Of the 12 ICUs, 9 agreed to participate and will be enrolled in the trial. Our primary outcome measure is the incidence of clinically relevant pDDIs per 1000 medication administrations.
Discussion: This study will identify pDDIs relevant for the ICU setting. It will also enhance our understanding of the effectiveness of alerts confined to clinically relevant pDDIs. Both of these contributions can facilitate the successful implementation of CDSSs in the ICU and in other domains as well.
Trial registration: Nederlands Trial register Identifier: NL6762 . Registered November 26, 2018.
Keywords: Alert fatigue; Computerized decision support systems; Drug-drug interactions; Intensive care; Stepped-wedge trial.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical