Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation
- PMID: 31409351
- PMCID: PMC6693148
- DOI: 10.1186/s12967-019-2013-1
Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation
Abstract
Background: Hypothermia, leading to mitochondrial inhibition, is widely used to reduce ischemic injury during kidney preservation. However, the exact effect of hypothermic kidney preservation on mitochondrial function remains unclear.
Methods: We evaluated mitochondrial function [i.e. oxygen consumption and production of reactive oxygen species (ROS)] in different models (porcine kidney perfusion, isolated kidney mitochondria, and HEK293 cells) at temperatures ranging 7-37 °C.
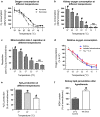
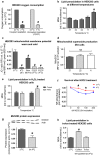
Results: Lowering temperature in perfused kidneys and isolated mitochondria resulted in a rapid decrease in oxygen consumption (65% at 27 °C versus 20% at 7 °C compared to normothermic). Decreased oxygen consumption at lower temperatures was accompanied by a reduction in mitochondrial ROS production, albeit markedly less pronounced and amounting only 50% of normothermic values at 7 °C. Consequently, malondialdehyde (a marker of ROS-induced lipid peroxidation) accumulated in cold stored kidneys. Similarly, low temperature incubation of kidney cells increased lipid peroxidation, which is due to a loss of ROS scavenging in the cold.
Conclusions: Lowering of temperature highly affects mitochondrial function, resulting in a progressive discrepancy between the lowering of mitochondrial respiration and their production of ROS, explaining the deleterious effects of hypothermia in transplantation procedures. These results highlight the necessity to develop novel strategies to decrease the formation of ROS during hypothermic organ preservation.
Keywords: Hypothermic preservation; Kidney transplantation; Machine perfusion; Mitochondrial function; Reactive oxygen species.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

