Pharmacological and Genetic Inhibition of Caveolin-1 Promotes Epithelialization and Wound Closure
- PMID: 31409528
- PMCID: PMC6838864
- DOI: 10.1016/j.ymthe.2019.07.016
Pharmacological and Genetic Inhibition of Caveolin-1 Promotes Epithelialization and Wound Closure
Abstract
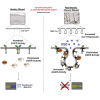
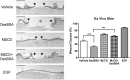
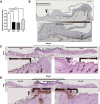
Chronic wounds-including diabetic foot ulcers, venous leg ulcers, and pressure ulcers-represent a major health problem that demands an urgent solution and new therapies. Despite major burden to patients, health care professionals, and health care systems worldwide, there are no efficacious therapies approved for treatment of chronic wounds. One of the major obstacles in achieving wound closure in patients is the lack of epithelial migration. Here, we used multiple pre-clinical wound models to show that Caveolin-1 (Cav1) impedes healing and that targeting Cav1 accelerates wound closure. We found that Cav1 expression is significantly upregulated in wound edge biopsies of patients with non-healing wounds, confirming its healing-inhibitory role. Conversely, Cav1 was absent from the migrating epithelium and is downregulated in acutely healing wounds. Specifically, Cav1 interacted with membranous glucocorticoid receptor (mbGR) and epidermal growth factor receptor (EGFR) in a glucocorticoid-dependent manner to inhibit cutaneous healing. However, pharmacological disruption of caveolae by MβCD or CRISPR/Cas9-mediated Cav1 knockdown resulted in disruption of Cav1-mbGR and Cav1-EGFR complexes and promoted epithelialization and wound healing. Our data reveal a novel mechanism of inhibition of epithelialization and wound closure, providing a rationale for pharmacological targeting of Cav1 as potential therapy for patients with non-healing chronic wounds.
Keywords: Caveolin-1; chronic wounds; diabetic foot ulcers; skin re-epithelialization; wound healing.
Copyright © 2019 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

