Chromatin occupancy and epigenetic analysis reveal new insights into the function of the GATA1 N terminus in erythropoiesis
- PMID: 31409672
- PMCID: PMC6871310
- DOI: 10.1182/blood.2019001234
Chromatin occupancy and epigenetic analysis reveal new insights into the function of the GATA1 N terminus in erythropoiesis
Abstract
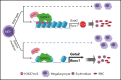
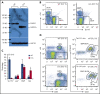
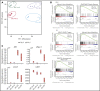
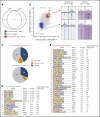
Mutations in GATA1, which lead to expression of the GATA1s isoform that lacks the GATA1 N terminus, are seen in patients with Diamond-Blackfan anemia (DBA). In our efforts to better understand the connection between GATA1s and DBA, we comprehensively studied erythropoiesis in Gata1s mice. Defects in yolks sac and fetal liver hematopoiesis included impaired terminal maturation and reduced numbers of erythroid progenitors. RNA-sequencing revealed that both erythroid and megakaryocytic gene expression patterns were altered by the loss of the N terminus, including aberrant upregulation of Gata2 and Runx1. Dysregulation of global H3K27 methylation was found in the erythroid progenitors upon loss of N terminus of GATA1. Chromatin-binding assays revealed that, despite similar occupancy of GATA1 and GATA1s, there was a striking reduction of H3K27me3 at regulatory elements of the Gata2 and Runx1 genes. Consistent with the observation that overexpression of GATA2 has been reported to impair erythropoiesis, we found that haploinsufficiency of Gata2 rescued the erythroid defects of Gata1s fetuses. Together, our integrated genomic analysis of transcriptomic and epigenetic signatures reveals that, Gata1 mice provide novel insights into the role of the N terminus of GATA1 in transcriptional regulation and red blood cell maturation which may potentially be useful for DBA patients.
© 2019 by The American Society of Hematology.
Figures
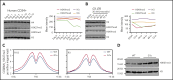
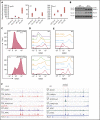
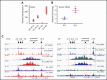
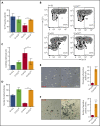
Comment in
-
The long and the short of it.Blood. 2019 Nov 7;134(19):1565-1566. doi: 10.1182/blood.2019002983. Blood. 2019. PMID: 31698422 Free PMC article. No abstract available.
References
-
- Balduini CL, Pecci A, Loffredo G, et al. . Effects of the R216Q mutation of GATA-1 on erythropoiesis and megakaryocytopoiesis. Thromb Haemost. 2004;91(1):129-140. - PubMed
-
- Freson K, Matthijs G, Thys C, et al. . Different substitutions at residue D218 of the X-linked transcription factor GATA1 lead to altered clinical severity of macrothrombocytopenia and anemia and are associated with variable skewed X inactivation. Hum Mol Genet. 2002;11(2):147-152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

