Muscle-generated BDNF is a sexually dimorphic myokine that controls metabolic flexibility
- PMID: 31409756
- PMCID: PMC7219567
- DOI: 10.1126/scisignal.aau1468
Muscle-generated BDNF is a sexually dimorphic myokine that controls metabolic flexibility
Abstract
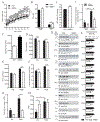
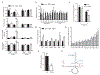
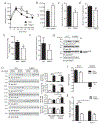
The ability of skeletal muscle to switch between lipid and glucose oxidation for ATP production during metabolic stress is pivotal for maintaining systemic energy homeostasis, and dysregulation of this metabolic flexibility is a dominant cause of several metabolic disorders. However, the molecular mechanism that governs fuel selection in muscle is not well understood. Here, we report that brain-derived neurotrophic factor (BDNF) is a fasting-induced myokine that controls metabolic reprograming through the AMPK/CREB/PGC-1α pathway in female mice. Female mice with a muscle-specific deficiency in BDNF (MBKO mice) were unable to switch the predominant fuel source from carbohydrates to fatty acids during fasting, which reduced ATP production in muscle. Fasting-induced muscle atrophy was also compromised in female MBKO mice, likely a result of autophagy inhibition. These mutant mice displayed myofiber necrosis, weaker muscle strength, reduced locomotion, and muscle-specific insulin resistance. Together, our results show that muscle-derived BDNF facilitates metabolic adaption during nutrient scarcity in a gender-specific manner and that insufficient BDNF production in skeletal muscle promotes the development of metabolic myopathies and insulin resistance.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Figures



References
-
- Randle PJ, Garland PB, Hales CN, Newsholme EA, The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 (1963). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

