Bounded rationality in C. elegans is explained by circuit-specific normalization in chemosensory pathways
- PMID: 31409788
- PMCID: PMC6692327
- DOI: 10.1038/s41467-019-11715-7
Bounded rationality in C. elegans is explained by circuit-specific normalization in chemosensory pathways
Abstract
Rational choice theory assumes optimality in decision-making. Violations of a basic axiom of economic rationality known as "Independence of Irrelevant Alternatives" (IIA) have been demonstrated in both humans and animals and could stem from common neuronal constraints. Here we develop tests for IIA in the nematode Caenorhabditis elegans, an animal with only 302 neurons, using olfactory chemotaxis assays. We find that in most cases C. elegans make rational decisions. However, by probing multiple neuronal architectures using various choice sets, we show that violations of rationality arise when the circuit of olfactory sensory neurons is asymmetric. We further show that genetic manipulations of the asymmetry between the AWC neurons can make the worm irrational. Last, a context-dependent normalization-based model of value coding and gain control explains how particular neuronal constraints on information coding give rise to irrationality. Thus, we demonstrate that bounded rationality could arise due to basic neuronal constraints.
Conflict of interest statement
The authors declare no competing interests.
Figures
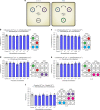
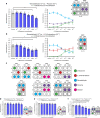
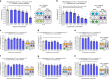
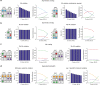
Similar articles
-
Irrational behavior in C. elegans arises from asymmetric modulatory effects within single sensory neurons.Nat Commun. 2019 Jul 19;10(1):3202. doi: 10.1038/s41467-019-11163-3. Nat Commun. 2019. PMID: 31324786 Free PMC article.
-
C. elegans odour discrimination requires asymmetric diversity in olfactory neurons.Nature. 2001 Apr 5;410(6829):698-701. doi: 10.1038/35070581. Nature. 2001. PMID: 11287957
-
Regulation of Diacylglycerol Content in Olfactory Neurons Determines Forgetting or Retrieval of Olfactory Memory in Caenorhabditis elegans.J Neurosci. 2022 Oct 26;42(43):8039-8053. doi: 10.1523/JNEUROSCI.0090-22.2022. Epub 2022 Sep 14. J Neurosci. 2022. PMID: 36104280 Free PMC article.
-
The cellular and genetic basis of olfactory responses in Caenorhabditis elegans.Ciba Found Symp. 1993;179:235-44; discussion 244-50. doi: 10.1002/9780470514511.ch15. Ciba Found Symp. 1993. PMID: 8168378 Review.
-
Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans.Annu Rev Genet. 1999;33:399-422. doi: 10.1146/annurev.genet.33.1.399. Annu Rev Genet. 1999. PMID: 10690413 Review.
Cited by
-
Divisive normalization does influence decisions with multiple alternatives.Nat Hum Behav. 2020 Nov;4(11):1118-1120. doi: 10.1038/s41562-020-00941-5. Epub 2020 Sep 14. Nat Hum Behav. 2020. PMID: 32929203 Free PMC article. No abstract available.
-
Decision neuroscience and neuroeconomics: Recent progress and ongoing challenges.Wiley Interdiscip Rev Cogn Sci. 2022 May;13(3):e1589. doi: 10.1002/wcs.1589. Epub 2022 Feb 8. Wiley Interdiscip Rev Cogn Sci. 2022. PMID: 35137549 Free PMC article. Review.
-
Calcium levels in ASER neurons determine behavioral valence by engaging distinct neuronal circuits in C. elegans.Nat Commun. 2025 Feb 20;16(1):1814. doi: 10.1038/s41467-025-57051-x. Nat Commun. 2025. PMID: 39979341 Free PMC article.
-
On the reliability of individual economic rationality measurements.Proc Natl Acad Sci U S A. 2022 Aug 2;119(31):e2202070119. doi: 10.1073/pnas.2202070119. Epub 2022 Jul 26. Proc Natl Acad Sci U S A. 2022. PMID: 35881803 Free PMC article.
-
Pre-acquired Functional Connectivity Predicts Choice Inconsistency.J Neurosci. 2024 May 1;44(18):e0453232024. doi: 10.1523/JNEUROSCI.0453-23.2024. J Neurosci. 2024. PMID: 38508713 Free PMC article.
References
-
- Afriat S. N. The Construction of Utility Functions from Expenditure Data. International Economic Review. 1967;8(1):67. doi: 10.2307/2525382. - DOI
-
- Kahneman D, Tversky A. Prospect theory—analysis of decision under. Risk. 1979;47:263–292.
-
- Tversky A, Kahneman D. Advances in prospect theory: cumulative representation of uncertainty. J. Risk Uncertain. 1992;5:297–323. doi: 10.1007/BF00122574. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

