MicroRNA-664 suppresses the growth of cervical cancer cells via targeting c-Kit
- PMID: 31409971
- PMCID: PMC6645611
- DOI: 10.2147/DDDT.S203399
MicroRNA-664 suppresses the growth of cervical cancer cells via targeting c-Kit
Abstract
Background: Cervical cancer is the second most common malignant cancer in women worldwide. Evidence indicated that miR-664 was significantly downregulated in cervical cancer. However, the mechanisms by which miR-664 regulates the tumorigenesis of cervical cancer remain unclear. Thus, this study aimed to investigate the role of miR-664 in cervical cancer.
Methods: Quantitative reverse transcription polymerase chain reaction was used to detect the level of miR-664 in tumor tissues and cell line. The dual luciferase reporter system assay and Western blotting were used to explore the interaction of miR-664 and c-Kit in cervical cancer.
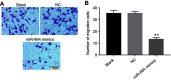
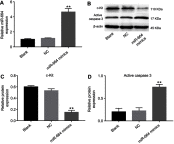
Results: The expression of miR-664 in patients with cervical cancer was dramatically decreased compared with that in adjacent tissues. MiR-664 mimics significantly inhibited proliferation in SiHa cells via inducing apoptosis. In addition, miR-664 mimics induced apoptosis in SiHa cells via increasing the expressions of Bax and active caspase 3 and decreasing the level of Bcl-2. Moreover, dual-luciferase assay showed that c-Kit was the directly binding target of miR-664 in SiHa cells; overexpression of miR-664 downregulated the expression of c-Kit. Meanwhile, upregulation of miR-664 significantly decreased the levels of c-Myc and Cyclin D in cells. Furthermore, miR-664 markedly inhibited tumor growth of cervical cancer in xenograft.
Conclusion: Our data indicated that miR-664 exerted antitumor effects on SiHa cells by directly targeting c-Kit in vitro and in vivo. Therefore, miR-664 might be a potential therapeutic target for the treatment of patients with cervical cancer.
Keywords: apoptosis; c-Kit; cervical cancer; microRNA-664.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. - PubMed
-
- de Freitas AC, Gomes Leitao Mda C, EC Coimbra. Prospects of molecularly-targeted therapies for cervical cancer treatment. Curr Drug Targets. 2015;16(1):77–91. - PubMed
-
- Shishodia G, Verma G, Das BC, Bharti AC. miRNA as viral transcription tuners in HPV-mediated cervical carcinogenesis. Front Biosci (Schol Ed). 2018;10:21–47. - PubMed
-
- Filippeschi M, Moncini I, Bianchi B, Florio P. What kind of surgery for cervical carcinoma? G Chir. 2012;33(4):139–146. - PubMed

