Construction and in vivo / in vitro evaluation of a nanoporous ion-responsive targeted drug delivery system for recombinant human interferon α-2b delivery
- PMID: 31409991
- PMCID: PMC6645601
- DOI: 10.2147/IJN.S209646
Construction and in vivo / in vitro evaluation of a nanoporous ion-responsive targeted drug delivery system for recombinant human interferon α-2b delivery
Erratum in
-
Erratum: Construction and in vivo/in vitro Evaluation of a Nanoporous Ion-Responsive Targeted Drug Delivery System for Recombinant Human Interferon α-2b Delivery [Corrigendum].Int J Nanomedicine. 2021 Jun 18;16:4191-4192. doi: 10.2147/IJN.S322682. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34168451 Free PMC article.
Abstract
Background: Like most protein macromolecular drugs, the half-life of rhIFNɑ-2b is short, with a low drug utilization rate, and the preparation and release conditions significantly affect its stability.
Methods: A nanoporous ion-responsive targeted drug delivery system (PIRTDDS) was designed to improve drug availability of rhIFNα-2b and target it to the lung passively with sustained release. Chitosan rhIFNα-2b carboxymethyl nanoporous microspheres (CS-rhIFNα-2b-CCPM) were prepared by the column method. Here, an electrostatic self-assembly technique was undertaken to improve and sustain rhIFNα-2b release rate.
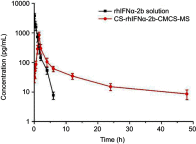
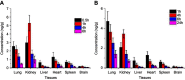
Results: The size distribution of the microspheres was 5~15 μm, and the microspheres contained nanopores 300~400 nm in diameter. The in vitro release results showed that rhIFNα-2b and CCPM were mainly bound by ionic bonds. After self-assembling, the release mechanism was transformed into being membrane diffusion. The accumulative release amount for 24 hrs was 83.89%. Results from circular dichrogram and SDS-PAGE electrophoresis showed that there was no significant change in the secondary structure and purity of rhIFNα-2b. Results from inhibition rate experiments for A549 cell proliferation showed that the antitumor activity of CS-rhIFNα-2b-CCPM for 24 hrs retained 91.98% of the stock solution, which proved that the drug-loaded nanoporous microspheres maintained good drug activity. In vivo pharmacokinetic experimental results showed that the drugs in CS-rhIFNα-2b-CCPM can still be detected in vivo after 24 hrs, equivalent to the stock solution at 6 hrs, which indicated that CS-rhIFNα-2b-CCPM had a certain sustained-release effect in vivo. The results of in vivo tissue distribution showed that CS-rhIFNα-2b-CCPM was mainly concentrated in the lungs of mice (1.85 times the stock solution). The pharmacodynamics results showed that CS-rhIFNα-2b-CCPM had an obvious antitumor effect, and the tumor inhibition efficiency was 29.2%.
Conclusion: The results suggested a novel sustained-release formulation with higher drug availability and better lung targeting from CS-rhIFNα-2b-CCPM compared to the reference (the stock solution of rhIFNα-2b), and, thus, should be further studied.
Keywords: ion exchange technique; ion-responsive; lung targeting; nanoporous; recombinant human interferon α-2b; sustained release.
Conflict of interest statement
There is no interest dispute between the project and the company that the authors were employed by. The authors report no other conflicts of interest in this work.
Figures






Similar articles
-
Establishment and In Vitro Evaluation of Porous Ion-Responsive Targeted Drug Delivery System.Protein Pept Lett. 2020;27(11):1102-1113. doi: 10.2174/0929866527666200320095453. Protein Pept Lett. 2020. PMID: 32196437
-
Preparation and Evaluation of rhINF-α-2b Sodium Hyaluronate Cross-Linked Porous Microspheres: Characterization, Sustained-Release Properties, and Antitumor Activity.AAPS PharmSciTech. 2021 Dec 20;23(1):31. doi: 10.1208/s12249-021-02178-5. AAPS PharmSciTech. 2021. PMID: 34931261
-
Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: in vitro and in vivo evaluation.Int J Mol Sci. 2014 Feb 26;15(3):3519-32. doi: 10.3390/ijms15033519. Int J Mol Sci. 2014. PMID: 24577314 Free PMC article.
-
Recent advances in microbeads-based drug delivery system for achieving controlled drug release.J Biomater Sci Polym Ed. 2023 Mar;34(4):541-564. doi: 10.1080/09205063.2022.2127237. Epub 2022 Oct 10. J Biomater Sci Polym Ed. 2023. PMID: 36168111 Review.
-
Long-Acting Strategies for Antibody Drugs: Structural Modification, Controlling Release, and Changing the Administration Route.Eur J Drug Metab Pharmacokinet. 2024 May;49(3):295-316. doi: 10.1007/s13318-024-00891-7. Epub 2024 Apr 18. Eur J Drug Metab Pharmacokinet. 2024. PMID: 38635015 Review.
Cited by
-
Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation.Vaccines (Basel). 2021 Apr 1;9(4):328. doi: 10.3390/vaccines9040328. Vaccines (Basel). 2021. PMID: 33915863 Free PMC article. Review.
-
The emergence of nanoporous materials in lung cancer therapy.Sci Technol Adv Mater. 2022 Jul 20;23(1):225-274. doi: 10.1080/14686996.2022.2052181. eCollection 2022. Sci Technol Adv Mater. 2022. PMID: 35875329 Free PMC article. Review.
-
Design of Decanoic Acid/Polysorbate 80 Composite Vesicles as Cosmetics Carrier: Stability, Skin Permeability, Antioxidant and Antibacterial Activity.Molecules. 2025 Jan 31;30(3):624. doi: 10.3390/molecules30030624. Molecules. 2025. PMID: 39942728 Free PMC article.
-
Influence of Lacidophilin Vaginal Capsules plus rh-IFN-α2b on Efficacy, Vaginal Microecology, and Safety of Patients with HPV Infection.Evid Based Complement Alternat Med. 2022 Aug 3;2022:3632053. doi: 10.1155/2022/3632053. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35966743 Free PMC article.
-
Pidotimod plus recombinant human interferon α-2b suppository boosts HPV clearance in high-risk patients following loop electrosurgical excision procedure.Am J Transl Res. 2025 Mar 15;17(3):2276-2282. doi: 10.62347/MLQC4007. eCollection 2025. Am J Transl Res. 2025. PMID: 40226023 Free PMC article.
References
-
- Hong WS, Jett JR, Sasaki Y, et al. Colony inhibitory effect of recombinant human tumor necrosis factor (rH-TNF) and/or recombinant human interferon (rH-IFN)-alpha, -beta and -gamma on human lung cancer cell lines. Jpn J Clin Oncol. 1987;17(1):49–55. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

