Fractionated photothermal therapy in a murine tumor model: comparison with single dose
- PMID: 31409993
- PMCID: PMC6645692
- DOI: 10.2147/IJN.S205409
Fractionated photothermal therapy in a murine tumor model: comparison with single dose
Abstract
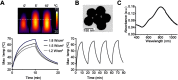
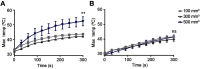
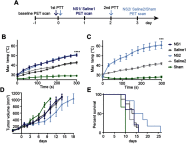
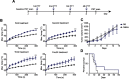
Purpose: Photothermal therapy (PTT) exploits the light-absorbing properties of nanomaterials such as silica-gold nanoshells (NS) to inflict tumor death through local hyperthermia. However, in in vivo studies of PTT, the heat distribution is often found to be heterogeneous throughout the tumor volume, which leaves parts of the tumor untreated and impairs the overall treatment outcome. As this challenges PTT as a one-dose therapy, this study here investigates if giving the treatment repeatedly, ie, fractionated PTT, increases the efficacy in mice bearing subcutaneous tumors. Methods: The NS heating properties were first optimized in vitro and in vivo. Two fractionated PTT protocols, consisting of two and four laser treatments, respectively, were developed and applied in a murine subcutaneous colorectal tumor model. The efficacy of the two fractionated protocols was evaluated both by longitudinal monitoring of tumor growth and, at an early time point, by positron emission tomography (PET) imaging of 18F-labeled glucose analog 18F-FDG. Results: Overall, there were no significant differences in tumor growth and survival between groups of mice receiving single-dose PTT and fractionated PTT in our study. Nonetheless, some animals did experience inhibited tumor growth or even complete tumor disappearance due to fractionated PTT, and these animals also showed a significant decrease in tumor uptake of 18F-FDG after therapy. Conclusion: This study only found an effect of giving PTT to tumors in fractions compared to a single-dose approach in a few animals. However, many factors can affect the outcome of PTT, and reliable tools for optimization of treatment protocol are needed. Despite the modest treatment effect, our results indicate that 18F-FDG PET/CT imaging can be useful to guide the number of treatment sessions necessary.
Keywords: cancer; fractionated therapy; hyperthermia; nanoparticle; photothermal therapy; positron emission tomography.
Conflict of interest statement
Ms Marina Simón, Dr Kamilla Norregaard, and Dr Jesper Tranekjær Jørgensen report grants from Novo Nordisk Foundation, European Research Council, Lundbeck Foundation, Innovation Fund Denmark, Danish Cancer Society, Arvid Nilsson Foundation, Svend Andersen Foundation, Neye Foundation, Research Foundation from Rigshospitalet, Research Council for Independent Research, Research Council of the Capital Region of Denmark, and Danish National Research Foundation, during the conduct of the study. Professor Lene Broeng Oddershede and Professor Andreas Kjaer report no conflicts of interest in this work.
Figures

Similar articles
-
18F-FDG positron emission tomography and diffusion-weighted magnetic resonance imaging for response evaluation of nanoparticle-mediated photothermal therapy.Sci Rep. 2020 May 5;10(1):7595. doi: 10.1038/s41598-020-64617-w. Sci Rep. 2020. PMID: 32371864 Free PMC article.
-
Nanoparticle-loaded macrophage-mediated photothermal therapy: potential for glioma treatment.Lasers Med Sci. 2015 May;30(4):1357-65. doi: 10.1007/s10103-015-1742-5. Epub 2015 Mar 21. Lasers Med Sci. 2015. PMID: 25794592 Free PMC article.
-
Evaluation of a nanocomposite of PEG-curcumin-gold nanoparticles as a near-infrared photothermal agent: an in vitro and animal model investigation.Lasers Med Sci. 2018 Nov;33(8):1769-1779. doi: 10.1007/s10103-018-2538-1. Epub 2018 May 22. Lasers Med Sci. 2018. PMID: 29790012
-
Recent advances in nanomaterials for enhanced photothermal therapy of tumors.Nanoscale. 2018 Dec 13;10(48):22657-22672. doi: 10.1039/c8nr07627h. Nanoscale. 2018. PMID: 30500042 Review.
-
In vitro outlook of gold nanoparticles in photo-thermal therapy: a literature review.Lasers Med Sci. 2018 May;33(4):917-926. doi: 10.1007/s10103-018-2467-z. Epub 2018 Feb 28. Lasers Med Sci. 2018. PMID: 29492712 Review.
Cited by
-
Neoadjuvant Gold Nanoshell-Based Photothermal Therapy Combined with Liposomal Doxorubicin in a Mouse Model of Colorectal Cancer.Int J Nanomedicine. 2023 Feb 17;18:829-841. doi: 10.2147/IJN.S389260. eCollection 2023. Int J Nanomedicine. 2023. PMID: 36824412 Free PMC article.
-
Application of Nanomaterials in Biomedical Imaging and Cancer Therapy.Nanomaterials (Basel). 2020 Aug 29;10(9):1700. doi: 10.3390/nano10091700. Nanomaterials (Basel). 2020. PMID: 32872399 Free PMC article. Review.
-
Application of MRI images based on Spatial Fuzzy Clustering Algorithm guided by Neuroendoscopy in the treatment of Tumors in the Saddle Region.Pak J Med Sci. 2021;37(6):1600-1604. doi: 10.12669/pjms.37.6-WIT.4850. Pak J Med Sci. 2021. PMID: 34712290 Free PMC article.
-
Anisotropic scattering characteristics of nanoparticles in different morphologies: improving the temperature uniformity of tumors during thermal therapy using forward scattering.Biomed Opt Express. 2021 Jan 15;12(2):893-906. doi: 10.1364/BOE.415666. eCollection 2021 Feb 1. Biomed Opt Express. 2021. PMID: 33680548 Free PMC article.
-
Nanoparticle-Mediated Photothermal Therapy Limitation in Clinical Applications Regarding Pain Management.Nanomaterials (Basel). 2022 Mar 10;12(6):922. doi: 10.3390/nano12060922. Nanomaterials (Basel). 2022. PMID: 35335735 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

