Probiotic effect on Helicobacter pylori attachment and inhibition of inflammation in human gastric epithelial cells
- PMID: 31410109
- PMCID: PMC6676116
- DOI: 10.3892/etm.2019.7742
Probiotic effect on Helicobacter pylori attachment and inhibition of inflammation in human gastric epithelial cells
Abstract
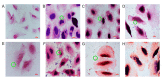
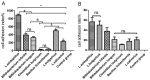
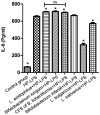
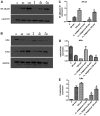
Helicobacter pylori (H. pylori) is a major cause of chronic gastritis, gastric ulcers and gastric cancer. Recent studies have identified that probiotics are beneficial to human health due, in part, to their anti-H. pylori activities. Therefore, the present study investigated the antagonistic and local immunoregulatory activities of seven commercial probiotic strains and explored their mechanisms of actions. The human gastric epithelial cell line-1 (GES-1) was used to assess the effects of probiotics on the adhesion ability of H. pylori. GES-1 cells were infected with H. pylori plus lipopolysaccharide (HP-LPS) or the drug-resistant H. pylori strain (HP021) in the presence or absence of live probiotics. Following this, the growth rate and the adhesion ability of GES-1 cells were detected using MTT and urease activity assay. Toll-like receptor 4 (TLR4), NFKB inhibitor-α (IκBα) and nuclear factor (NF)-κB levels were measured by western blot analysis. The amount of interleukin (IL)-8 in the cell culture medium was determined by ELISA. Amongst the seven probiotic strains studied, live Lactobacillus acidophilus (L. acidophilus) and Lactobacillus bulgaricus (L. bulgaricus) inhibited H. pylori adherence to GES-1 cells most significantly. L. bulgaricus inhibited IL-8 production by GES-1 cells through modulation of the TLR4/IκBα/NF-κB pathway. Therefore, the present results suggested that consumption of food containing L. acidophilus and L. bulgaricus may be used as an adjuvant therapy for H. pylori-associated gastritis.
Keywords: Helicobacter pylori; cell adhesion; inflammation; lipopolysaccharide; probiotics.
Figures




Similar articles
-
Probiotic BIFICO cocktail ameliorates Helicobacter pylori induced gastritis.World J Gastroenterol. 2015 Jun 7;21(21):6561-71. doi: 10.3748/wjg.v21.i21.6561. World J Gastroenterol. 2015. PMID: 26074694 Free PMC article.
-
[Effect of Weifuchun on inhibiting inflammation of Helicobacter pylori-infected GES-1 cells and NF-kappaB signaling pathway].Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014 Apr;34(4):450-4. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014. PMID: 24812903 Chinese.
-
Lactobacillus acidophilus NCFM and Lactiplantibacillus plantarum Lp-115 inhibit Helicobacter pylori colonization and gastric inflammation in a murine model.Front Cell Infect Microbiol. 2023 Aug 9;13:1196084. doi: 10.3389/fcimb.2023.1196084. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37621875 Free PMC article.
-
[Use of probiotics and probiotic-based immunomodulators as adjuvant therapy for Helicobacter pylori eradication].Ter Arkh. 2016;88(12):140-148. doi: 10.17116/terarkh20168812140-148. Ter Arkh. 2016. PMID: 28635890 Review. Russian.
-
Clinical application of probiotics in the treatment of Helicobacter pylori infection--a brief review.J Microbiol Immunol Infect. 2014 Oct;47(5):429-37. doi: 10.1016/j.jmii.2013.03.010. Epub 2013 Jun 10. J Microbiol Immunol Infect. 2014. PMID: 23757373 Review.
Cited by
-
The Influence of Gastric Microbiota and Probiotics in Helicobacter pylori Infection and Associated Diseases.Biomedicines. 2024 Dec 30;13(1):61. doi: 10.3390/biomedicines13010061. Biomedicines. 2024. PMID: 39857645 Free PMC article. Review.
-
Immunomodulatory effects of live and pasteurized Lactobacillus crispatus strain RIGLD-1 on Helicobacter pylori-triggered inflammation in gastric epithelial cells in vitro.Mol Biol Rep. 2023 Aug;50(8):6795-6805. doi: 10.1007/s11033-023-08596-x. Epub 2023 Jul 1. Mol Biol Rep. 2023. PMID: 37392285
-
Interplay and cooperation of Helicobacter pylori and gut microbiota in gastric carcinogenesis.BMC Microbiol. 2021 Sep 23;21(1):258. doi: 10.1186/s12866-021-02315-x. BMC Microbiol. 2021. PMID: 34556055 Free PMC article. Review.
-
Probiotics and prebiotics: new treatment strategies for oral potentially malignant disorders and gastrointestinal precancerous lesions.NPJ Biofilms Microbiomes. 2025 Apr 8;11(1):55. doi: 10.1038/s41522-025-00688-9. NPJ Biofilms Microbiomes. 2025. PMID: 40199865 Free PMC article. Review.
-
Supernatants from Newly Isolated Lacticaseibacillus paracasei P4 Ameliorate Adipocyte Metabolism in Differentiated 3T3-L1 Cells.Biomedicines. 2024 Dec 7;12(12):2785. doi: 10.3390/biomedicines12122785. Biomedicines. 2024. PMID: 39767692 Free PMC article.
References
-
- Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
