Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617
- PMID: 31410185
- PMCID: PMC6691377
- DOI: 10.7150/thno.35759
Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617
Abstract
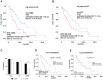
The purpose of this study was to identify previous treatments and biomarker profile features that prognosticate overall survival (OS) in patients with mCRPC receiving 177Lu-PSMA-617. Methods: 109 mCRPC patients treated with a median of 3 cycles of 177Lu-PSMA-617 were included. Data were analyzed according to OS as well as PSA response patterns with regard to prior therapies, laboratory biomarkers and metastatic extent in univariate as well as multivariate Cox's proportional hazards models. PSA decline was assessed using the lowest PSA levels after the first cycle of therapy (initial PSA response) and during the entire observation period (best PSA response). Results: In total, 54 patients (49.5%) died during the observation period. First and second line chemotherapy were performed in 85% and 26%, and Abiraterone and Enzalutamide were administered in 83% and 85%, respectively. Any initial PSA decline occurred in 55% while 25% showed a PSA decline of ≥50%. The median estimated OS was 9.9 months (95% CI: 7.2-12.5) for all patients. Any initial decline of PSA was associated with significantly prolonged OS (15.5 vs. 5.7 months, p = 0.002). Second line cabazitaxel chemotherapy (6.7 vs. 15.7 months, p = 0.002) and presence of visceral metastases (5.9 vs. 16.4 months, p<0.001) were associated with shorter OS. Only visceral metastases remained significant in a multivariate analysis. Conclusion:177Lu-PSMA-617 is an effective therapy for patients with mCRPC. However, the present data indicate that its beneficial effects on OS are strongly influenced by pretreatment (history of second line chemotherapy with cabazitaxel) and the presence of visceral metastases at onset of 177Lu-PSMA-617 treatment.
Keywords: 177Lu-PSMA-617; PSMA; cabazitaxel; mCRPC; metastases; prostate cancer; radioligand therapy; second line chemotherapy.
Conflict of interest statement
Competing Interests: The University of Münster received consulting fees from ABX GmbH, Radeberg, Germany for K.R. and M.B. Additionally K.R. is scientific consultant/ advisor of ABX GmbH. The authors declare they have no conflict of interest according to the subject and matter of the present article.
Figures


Comment in
-
Metastatic extent predicts survival as patients with metastatic castration-resistant prostate cancer are treated with 177Lu-PSMA radioligand therapy.Theranostics. 2020 Mar 30;10(11):4900-4902. doi: 10.7150/thno.44568. eCollection 2020. Theranostics. 2020. PMID: 32308757 Free PMC article.
References
-
- Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M. et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med. 2017;58:85–90. - PubMed
-
- Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T. et al. [Lu-177]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

