Identification of novel antifungal agents: antimicrobial evaluation, SAR, ADME-Tox and molecular docking studies of a series of imidazole derivatives
- PMID: 31410411
- PMCID: PMC6685181
- DOI: 10.1186/s13065-019-0623-6
Identification of novel antifungal agents: antimicrobial evaluation, SAR, ADME-Tox and molecular docking studies of a series of imidazole derivatives
Abstract
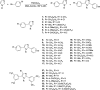
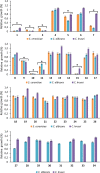
Thirty-four imidazole-based compounds synthesized by one-pot catalytic method were evaluated for their antifungal and antibacterial activities against several fungal and bacterial strains. None of the compounds had antibacterial activity. Interestingly, compounds 1, 2, 3, 10 and 15 displayed a strong antifungal activity against all the tested fungal species, while compounds 5, 7, 9, 11, 21 and 27 showed a moderate antifungal activity. To better understand the biological activity of the most active compounds ADME-Tox and molecular docking studies were carried out. Interestingly, compounds 1, 2, 3, 7, 10 and 15 showed excellent bioavailability. In addition, compounds 1, 2 and 3, exhibited good toxicity profiles. Docking studies of the two most active compounds 2 (IC50 of 95 ± 7.07 μM) and 10 (IC50 of 235 ± 7.07 μM) suggested that they might act by inhibiting the fungal lanosterol 14α-demethylase. Therefore, these novel antifungal agents merit further characterization for the development of new antifungal therapeutics.
Keywords: ADME–Tox; Antibacterial; Antifungal; Docking; Imidazole; Structure–activity relationship.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures

Similar articles
-
Design, Synthesis and Antifungal Activity Evaluation of New Thiazolin-4-ones as Potential Lanosterol 14α-Demethylase Inhibitors.Int J Mol Sci. 2017 Jan 17;18(1):177. doi: 10.3390/ijms18010177. Int J Mol Sci. 2017. PMID: 28106743 Free PMC article.
-
In silico and POM analysis for potential antimicrobial agents of thymidine analogs by using molecular docking, molecular dynamics and ADMET profiling.Nucleosides Nucleotides Nucleic Acids. 2023;42(11):877-918. doi: 10.1080/15257770.2023.2215839. Epub 2023 May 26. Nucleosides Nucleotides Nucleic Acids. 2023. PMID: 37235455
-
Synthesis, antimicrobial, SAR, PASS, molecular docking, molecular dynamics and pharmacokinetics studies of 5'-O-uridine derivatives bearing acyl moieties: POM study and identification of the pharmacophore sites.Nucleosides Nucleotides Nucleic Acids. 2022;41(10):1036-1083. doi: 10.1080/15257770.2022.2096898. Epub 2022 Jul 7. Nucleosides Nucleotides Nucleic Acids. 2022. PMID: 35797068
-
Azole Derivatives: Recent Advances as Potent Antibacterial and Antifungal Agents.Curr Med Chem. 2023;30(2):220-249. doi: 10.2174/0929867329666220407094430. Curr Med Chem. 2023. PMID: 35392780 Review.
-
Synthetic Imidazopyridine-Based Derivatives as Potential Inhibitors against Multi-Drug Resistant Bacterial Infections: A Review.Antibiotics (Basel). 2022 Nov 22;11(12):1680. doi: 10.3390/antibiotics11121680. Antibiotics (Basel). 2022. PMID: 36551338 Free PMC article. Review.
Cited by
-
Selective photocatalytic oxidation of aromatic alcohols to aldehydes with air by magnetic WO3ZnO/Fe3O4. In situ photochemical synthesis of 2-substituted benzimidazoles.RSC Adv. 2020 Nov 9;10(67):40725-40738. doi: 10.1039/d0ra08403d. eCollection 2020 Nov 9. RSC Adv. 2020. PMID: 35519184 Free PMC article.
-
New N-Alkylated Heterocyclic Compounds as Prospective NDM1 Inhibitors: Investigation of In Vitro and In Silico Properties.Pharmaceuticals (Basel). 2022 Jun 28;15(7):803. doi: 10.3390/ph15070803. Pharmaceuticals (Basel). 2022. PMID: 35890102 Free PMC article.
-
In silico Approaches for Exploring the Pharmacological Activities of Benzimidazole Derivatives: A Comprehensive Review.Mini Rev Med Chem. 2024;24(16):1481-1495. doi: 10.2174/0113895575287322240115115125. Mini Rev Med Chem. 2024. PMID: 38288816 Review.
References
-
- The top 10 causes of death. World Health Organization. 2018. http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-.... Accessed 13 Feb 2019
LinkOut - more resources
Full Text Sources
Miscellaneous
