Existing and Emerging Biomarkers for Immune Checkpoint Immunotherapy in Solid Tumors
- PMID: 31410780
- PMCID: PMC6778545
- DOI: 10.1007/s12325-019-01051-z
Existing and Emerging Biomarkers for Immune Checkpoint Immunotherapy in Solid Tumors
Abstract
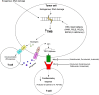
In the last few years, immunotherapy has transformed the way we treat solid tumors, including melanoma, lung, head neck, breast, renal, and bladder cancers. Durable responses and long-term survival benefit has been experienced by many cancer patients, with favorable toxicity profiles of immunotherapeutic agents relative to chemotherapy. Cures have become possible in some patients with metastatic disease. Additional approvals of immunotherapy drugs and in combination with other agents are anticipated in the near future. Multiple additional immunotherapy drugs are in earlier stages of clinical development, and their testing in additional tumor types is under way. Despite considerable early success and relatively fewer side effects, the majority of cancer patients do not respond to checkpoint inhibitors. Additionally, while the drugs are generally well tolerated, there is still the potential for significant, unpredictable and even fatal toxicity with these agents. Improved biomarkers may help to better select patients who are more likely to respond to these drugs. Two key biologically important predictive tissue biomarkers, specifically, PD-L1 and mismatch repair deficiency, have been FDA-approved in conjunction with the checkpoint inhibitor, pembrolizumab. Tumor mutation burden, another promising biomarker, is emerging in several tumor types, and may also soon receive approval. Finally, several other tissue and liquid biomarkers are emerging that could help guide single-agent immunotherapy and in combination with other agents. Of these, one promising investigational biomarker is alteration or deficiency in DNA damage response (DDR) pathways, with altered DDR observed in a broad spectrum of tumors. Here, we provide a critical overview of current, emerging, and investigational biomarkers in the context of response to immunotherapy in solid tumors.
Keywords: Biomarkers; Cytotoxic T-lymphocyte antigen 4 (CTLA-4); DNA damage response (DDR); Immunotherapy; Mismatch repair deficiency (MMR); Programmed death 1 (PD-1); Tumor mutation burden (TMB).
Figures

References
-
- Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, Mistry A, Kalamegham R, Averbuch S, Novotny J, Rubin E, Emancipator K, McCaffery I, Williams JA, Walker J, Longshore J, Tsao MS, Kerr KM. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208–222. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

