Kidney dopamine D1-like receptors and angiotensin 1-7 interaction inhibits renal Na+ transporters
- PMID: 31411069
- PMCID: PMC6843046
- DOI: 10.1152/ajprenal.00135.2019
Kidney dopamine D1-like receptors and angiotensin 1-7 interaction inhibits renal Na+ transporters
Abstract
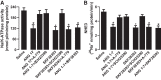
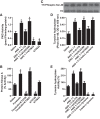
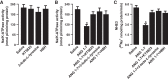
The role of dopamine D1-like receptors (DR) in the regulation of renal Na+ transporters, natriuresis, and blood pressure is well established. However, the involvement of the angiotensin 1-7 (ANG 1-7)-Mas receptor in the regulation of Na+ balance and blood pressure is not clear. The present study aimed to investigate the hypothesis that ANG 1-7 can regulate Na+ homeostasis by modulating the renal dopamine system. Sprague-Dawley rats were infused with saline alone (vehicle) or saline with ANG 1-7, ANG 1-7 antagonist A-779, DR agonist SKF38393, and antagonist SCH23390. Infusion of ANG 1-7 caused significant natriuresis and diuresis compared with saline alone. Both natriuresis and diuresis were blocked by A-779 and SCH23390. SKF38393 caused a significant, SCH23390-sensitive natriuresis and diuresis, and A-779 had no effect on the SKF38393 response. Concomitant infusion of ANG 1-7 and SKF38393 did not show a cumulative effect compared with either agonist alone. Treatment of renal proximal tubules with ANG 1-7 or SKF38393 caused a significant decrease in Na+-K+-ATPase and Na+/H+ exchanger isoform 3 activity. While SCH23390 blocked both ANG 1-7- and SKF38393-induced inhibition, the DR response was not sensitive to A-779. Additionally, ANG 1-7 activated PKG, enhanced tyrosine hydroxylase activity via Ser40 phosphorylation, and increased renal dopamine production. These data suggest that ANG 1-7, via PKG, enhances tyrosine hydroxylase activity, which increases renal dopamine production and activation of DR and subsequent natriuresis. This study provides evidence for a unidirectional functional interaction between two G protein-coupled receptors to regulate renal Na+ transporters and induce natriuresis.
Keywords: Na+-K+-ATPase; Na+/H+ exchanger; natriuresis; renal tubules.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
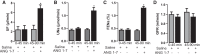

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

