Phase II Trial of De-Intensified Chemoradiotherapy for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma
- PMID: 31411949
- PMCID: PMC7010421
- DOI: 10.1200/JCO.19.01007
Phase II Trial of De-Intensified Chemoradiotherapy for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma
Abstract
Purpose: To report the results of a phase II clinical trial of de-intensified chemoradiotherapy for patients with human papillomavirus-associated oropharyngeal squamous cell carcinoma.
Materials and methods: Major inclusion criteria were (1) having American Joint Committee on Cancer (AJCC) 7th edition T0-T3, N0-N2c, M0 (AJCC 8th edition T0-T3, N0-N2, M0), (2) being p16 positive, and (3) reporting minimal or remote smoking history. Treatment was limited to 60 Gy intensity-modulated radiotherapy with concurrent intravenous cisplatin 30 mg/m2 once per week. Patients with T0-T2 N0-1 (AJCC 7th edition) did not receive chemotherapy. All patients had a 10- to 12-week post-treatment positron emission tomography/computed tomography to assess for neck dissection. The primary end point was 2-year progression-free survival. Secondary end points included 2-year local-regional control, distant metastasis-free survival and overall survival, and patient-reported outcomes (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire and the patient-reported outcomes version of the Common Terminology Criteria for Adverse Events).
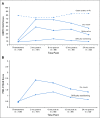
Results: One hundred fourteen patients were enrolled (median follow-up of 31.8 months), with 81% having a minimum follow-up of 2 years. Eighty percent of patients had 10 or fewer tobacco pack-years. Two-year local-regional control, distant metastasis-free survival, progression-free survival, and overall survival were as follows: 95%, 91%, 86%, and 95%, respectively. Mean pre- and 2-year post-treatment European Organisation for Research and Treatment of Cancer quality of life scores were as follows: global, 79/84 (lower worse); swallowing, 8/9 (higher worse); and dry mouth, 14/45 (higher worse). Mean pre- and 2-year post-treatment patient-reported outcomes version of the Common Terminology Criteria for Adverse Events scores (0 to 4 scale, higher worse) were as follows: swallowing, 0.5/0.7, and dry mouth, 0.4/1.3. Thirty-four percent of patients required a feeding tube (median, 10.5 weeks; none permanent). There were no grade 3 or higher late adverse events.
Conclusion: Clinical outcomes with a de-intensified chemoradiotherapy regimen of 60 Gy intensity-modulated radiotherapy with concurrent low-dose cisplatin are favorable in patients with human papillomavirus-associated oropharyngeal squamous cell carcinoma. Neither neoadjuvant chemotherapy nor routine surgery is needed to obtain favorable results with de-escalation.
Trial registration: ClinicalTrials.gov NCT02281955.
Figures

Comment in
-
Four Influential Clinical Trials in Human Papilloma Virus-Associated Oropharynx Cancer.Int J Radiat Oncol Biol Phys. 2020 Apr 1;106(5):893-899. doi: 10.1016/j.ijrobp.2019.12.015. Int J Radiat Oncol Biol Phys. 2020. PMID: 32171456 No abstract available.
References
-
- Reference deleted.
-
- ECOG-ACRIN: E3311 – Phase II randomized trial of transoral surgical resection followed by low-dose or standard-dose IMRT in resectable p16+ locally advanced oropharynx cancer. http://ecog-acrin.org/clinical-trials/e3311-educational-materials. - PMC - PubMed
-
- Cmelak A, Li S, Marur S, et al: E1308: Reduced-dose IMRT in human papilloma virus (HPV)-associated resectable oropharyngeal squamous carcinomas (OPSCC) after clinical complete response (cCR) to induction chemotherapy (IC). J Clin Oncol [epub ahead of print on January 31, 2017]
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

