Incidence, Patterns, and Outcomes with Adjuvant Chemotherapy for Residual Disease After Neoadjuvant Chemotherapy in Muscle-invasive Urinary Tract Cancers
- PMID: 31411990
- PMCID: PMC8449851
- DOI: 10.1016/j.euo.2018.12.013
Incidence, Patterns, and Outcomes with Adjuvant Chemotherapy for Residual Disease After Neoadjuvant Chemotherapy in Muscle-invasive Urinary Tract Cancers
Abstract
Background: Patients with residual muscle-invasive urinary tract cancer after neoadjuvant chemotherapy (NAC) have a high risk of recurrence.
Objective: To retrospectively evaluate whether additional adjuvant chemotherapy (AC) improves outcomes compared with surveillance in patients with significant residual disease despite NAC.
Design, setting, and participants: We identified 474 patients who received NAC from the Retrospective International Study of Cancers of the Urothelium database, of whom 129 had adverse residual disease (≥ypT3 and/or ypN+).
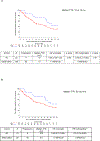
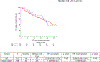
Outcome measurements and statistical analysis: Time to relapse (TTR) was the primary endpoint assessed starting from 2mo after surgery to minimize immortal time bias. Secondary endpoints included overall survival (OS), incidence of AC use, and chemotherapy patterns. Kaplan-Meier and Cox regression models estimated TTR, OS, and associations with AC, adjusting for the type of NAC, age, and pathological stage in multivariable analyses.
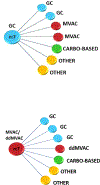
Results and limitations: A total of 106 patients underwent surveillance, while 23 received AC. Gemcitabine-cisplatin was the most frequent regimen employed in both settings (30.4%), and the majority (82.6%) of the patients switched to a different regimen. Median follow-up was 30mo. Over 50% of patients developed a recurrence. Median TTR was 16mo (range: <1-108mo). Longer median TTR was observed with AC compared with surveillance (18 vs 10mo, p=0.06). Risk of relapse significantly decreased with AC when adjusted in multivariable analyses (p=0.01). The subgroup analyses of ypT4b/ypN+ patients (AC: 19; surveillance: 50) who received AC had significantly greater median TTR (20 vs 9mo; hazard ratio 0.43; 95% confidence interval: 0.21-0.89). No difference in OS was found. Limitations include the retrospective design.
Conclusions: The utilization of AC after NAC in patients with high-risk residual disease is not frequent in clinical practice but might reduce the risk of recurrence. Further investigation is needed in this high-risk population to identify optimal therapy and to improve clinical outcomes such as the ongoing adjuvant immunotherapy trials.
Patient summary: We found that administering additional chemotherapy in patients who had significant residual disease despite preoperative chemotherapy is not frequent in clinical practice. While it might reduce the risk of recurrence, it did not clearly increase overall survival. We encourage participation in the ongoing immunotherapy trials to see whether we can improve outcomes using a different type of therapy that stimulates the immune system.
Keywords: Adjuvant chemotherapy; Muscle-invasive bladder cancer; Muscle-invasive urinary tract cancer; Neoadjuvant chemotherapy; Residual disease; Risk of relapse; Time to recurrence.
Copyright © 2019. Published by Elsevier B.V.
Figures
References
-
- Flaig TW, Spiess PE, Agarwal N, et al.NCCN guidelines insights: bladder cancer version 5. J Natl Compr Canc Netw 2018;16:1041–53. - PubMed
-
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202–5; discussion 205–6. - PubMed
-
- International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organization for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, et al.International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171–7. - PMC - PubMed
-
- Plimack ER, Hoffman-Censits JH, Viterbo R, et al.Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

