A cryptic non-GPI-anchored cytosolic isoform of CD59 controls insulin exocytosis in pancreatic β-cells by interaction with SNARE proteins
- PMID: 31412214
- PMCID: PMC6902737
- DOI: 10.1096/fj.201901007R
A cryptic non-GPI-anchored cytosolic isoform of CD59 controls insulin exocytosis in pancreatic β-cells by interaction with SNARE proteins
Abstract
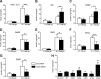
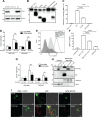
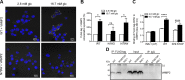
CD59 is a glycosylphosphatidylinositol (GPI)-anchored cell surface inhibitor of the complement membrane attack complex (MAC). We showed previously that CD59 is highly expressed in pancreatic islets but is down-regulated in rodent models of diabetes. CD59 knockdown but not enzymatic removal of cell surface CD59 led to a loss of glucose-stimulated insulin secretion (GSIS), suggesting that an intracellular pool of CD59 is required. In this current paper, we now report that non-GPI-anchored CD59 is present in the cytoplasm, colocalizes with exocytotic protein vesicle-associated membrane protein 2, and completely rescues GSIS in cells lacking endogenous CD59 expression. The involvement of cytosolic non-GPI-anchored CD59 in GSIS is supported in phosphatidylinositol glycan class A knockout GPI anchor-deficient β-cells, in which GSIS is still CD59 dependent. Furthermore, site-directed mutagenesis demonstrated different structural requirements of CD59 for its 2 functions, MAC inhibition and GSIS. Our results suggest that CD59 is retrotranslocated from the endoplasmic reticulum to the cytosol, a process mediated by recognition of trimmed N-linked oligosaccharides, supported by the partial glycosylation of non-GPI-anchored cytosolic CD59 as well as the failure of N-linked glycosylation site mutant CD59 to reach the cytosol or rescue GSIS. This study thus proposes the previously undescribed existence of non-GPI-anchored cytosolic CD59, which is required for insulin secretion.-Golec, E., Rosberg, R., Zhang, E., Renström, E., Blom, A. M., King, B. C. A cryptic non-GPI-anchored cytosolic isoform of CD59 controls insulin exocytosis in pancreatic β-cells by interaction with SNARE proteins.
Keywords: CD59 isoforms; VAMP2; diabetes; glycosylphosphatidylinositol; insulin secretion.
Conflict of interest statement
This work was funded by the Knut and Alice Wallenberg Foundation (to A.B. and E.R.); Diabetesfonden (to A.B.); and the Greta and Johan Kock’s Foundation, the Royal Physiographical Society in Lund, the Lars Hiertas Memorial Fund, the Albert Påhlsson Fund, the Crafoord Foundation, and the Tore Nilssons Foundation (all to B.C.K.). Funders played no role in study design or execution. The authors declare no conflicts of interest.
Figures


References
-
- King B. C., Kulak K., Krus U., Rosberg R., Golec E., Wozniak K., Gomez M. F., Zhang E., O’Connell D. J., Renstrom E., Blom A. M. (2019) Complement component C3 is highly expressed in human pancreatic islets and prevents β cell death via ATG16L1 interaction and autophagy regulation. Cell Metab. 29, 202–210.e6 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

