m6A RNA Methylation Maintains Hematopoietic Stem Cell Identity and Symmetric Commitment
- PMID: 31412241
- PMCID: PMC6818972
- DOI: 10.1016/j.celrep.2019.07.032
m6A RNA Methylation Maintains Hematopoietic Stem Cell Identity and Symmetric Commitment
Abstract
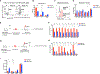
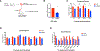
Stem cells balance cellular fates through asymmetric and symmetric divisions in order to self-renew or to generate downstream progenitors. Symmetric commitment divisions in stem cells are required for rapid regeneration during tissue damage and stress. The control of symmetric commitment remains poorly defined. Using single-cell RNA sequencing (scRNA-seq) in combination with transcriptomic profiling of HSPCs (hematopoietic stem and progenitor cells) from control and m6A methyltransferase Mettl3 conditional knockout mice, we found that m6A-deficient hematopoietic stem cells (HSCs) fail to symmetrically differentiate. Dividing HSCs are expanded and are blocked in an intermediate state that molecularly and functionally resembles multipotent progenitors. Mechanistically, RNA methylation controls Myc mRNA abundance in differentiating HSCs. We identified MYC as a marker for HSC asymmetric and symmetric commitment. Overall, our results indicate that RNA methylation controls symmetric commitment and cell identity of HSCs and may provide a general mechanism for how stem cells regulate differentiation fate choice.
Keywords: MYC; RNA methylation; cell identity; hematopoietic stem cell; m(6)A; symmetric and asymmetric cell division.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Attar EC, and Scadden DT (2004). Regulation of hematopoietic stem cell growth. Leukemia 18, 1760–1768. - PubMed
-
- Bahr C, von Paleske L, Uslu VV, Remeseiro S, Takayama N, Ng SW, Murison A, Langenfeld K, Petretich M, Scognamiglio R, et al. (2018). A Myc enhancer cluster regulates normal and leukaemic haematopoietic stem cell hierarchies. Nature 553, 515–520. - PubMed
-
- Boriack-Sjodin PA, Ribich S, and Copeland RA (2018). RNA-modifying proteins as anticancer drug targets. Nat. Rev. Drug Discov. 17, 435–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

