Complex Economic Behavior Patterns Are Constructed from Finite, Genetically Controlled Modules of Behavior
- PMID: 31412249
- PMCID: PMC7476553
- DOI: 10.1016/j.celrep.2019.07.038
Complex Economic Behavior Patterns Are Constructed from Finite, Genetically Controlled Modules of Behavior
Abstract
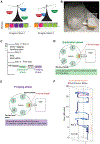
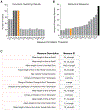
Complex ethological behaviors could be constructed from finite modules that are reproducible functional units of behavior. Here, we test this idea for foraging and develop methods to dissect rich behavior patterns in mice. We uncover discrete modules of foraging behavior reproducible across different strains and ages, as well as nonmodular behavioral sequences. Modules differ in terms of form, expression frequency, and expression timing and are expressed in a probabilistically determined order. Modules shape economic patterns of feeding, exposure, activity, and perseveration responses. The modular architecture of foraging changes developmentally, and different developmental, genetic, and parental effects are found to shape the expression of specific modules. Dissecting modules from complex patterns is powerful for phenotype analysis. We discover that both parental alleles of the imprinted Prader-Willi syndrome gene Magel2 are functional in mice but regulate different modules. Our study found that complex economic patterns are built from finite, genetically controlled modules.
Keywords: Prader-Willi syndrome; behavior; epigenetics; foraging; genomic imprinting; machine learning; neuroeconomics; neuroscience.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare that a patent on this work is pending.
Figures





References
-
- Anderson DJ, and Perona P (2014). Toward a science of computational ethology. Neuron 84, 18–31. - PubMed
-
- Bervini S, and Herzog H (2013). Mouse models of Prader-Willi syndrome: a systematic review. Front. Neuroendocrinol 34, 107–119. - PubMed
-
- Bonthuis PJ, Huang W-C, Stacher Hörndli CN, Ferris E, Cheng T, and Gregg C (2015). Noncanonical Genomic Imprinting Effects in Offspring. Cell Rep. 12, 979–991. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

