Scleral structure and biomechanics
- PMID: 31412277
- PMCID: PMC7187923
- DOI: 10.1016/j.preteyeres.2019.100773
Scleral structure and biomechanics
Abstract
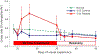
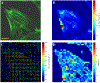
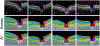
As the eye's main load-bearing connective tissue, the sclera is centrally important to vision. In addition to cooperatively maintaining refractive status with the cornea, the sclera must also provide stable mechanical support to vulnerable internal ocular structures such as the retina and optic nerve head. Moreover, it must achieve this under complex, dynamic loading conditions imposed by eye movements and fluid pressures. Recent years have seen significant advances in our knowledge of scleral biomechanics, its modulation with ageing and disease, and their relationship to the hierarchical structure of the collagen-rich scleral extracellular matrix (ECM) and its resident cells. This review focuses on notable recent structural and biomechanical studies, setting their findings in the context of the wider scleral literature. It reviews recent progress in the development of scattering and bioimaging methods to resolve scleral ECM structure at multiple scales. In vivo and ex vivo experimental methods to characterise scleral biomechanics are explored, along with computational techniques that combine structural and biomechanical data to simulate ocular behaviour and extract tissue material properties. Studies into alterations of scleral structure and biomechanics in myopia and glaucoma are presented, and their results reconciled with associated findings on changes in the ageing eye. Finally, new developments in scleral surgery and emerging minimally invasive therapies are highlighted that could offer new hope in the fight against escalating scleral-related vision disorder worldwide.
Keywords: Ageing; Biomechanics; Connective tissue structure; Glaucoma; Myopia; Sclera.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


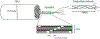


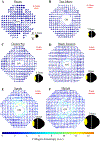

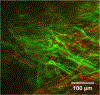
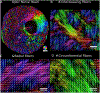
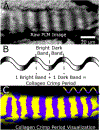


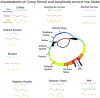







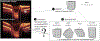






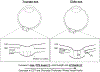
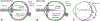




References
-
- Albon J, Farrant S, Akhtar S, Young R, Boulton ME, Smith G, Taylor M, Guggenheim J, Morgan JE, 2007. Connective tissue structure of the tree shrew optic nerve and associated ageing changes. Invest Ophthalmol Vis Sci. 48, 2134–2144. - PubMed
-
- Anderson DR, 1969. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol 82, 800–814. - PubMed
-
- Ashby R, Ohlendorf A, Schaeffel F, 2009. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci 50, 5348–5354. - PubMed
-
- Austin BA, Coulon C, Liu CY, Kao WW, Rada JA, 2002. Altered collagen fibril formation in the sclera of lumican-deficient mice. Invest Ophthalmol Vis Sci 43, 1695–1701. - PubMed
-
- Ayyalasomayajula A, Park RI, Simon BR, Vande Geest JP, 2016. A porohyperelastic finite element model of the eye: the influence of stiffness and permeability on intraocular pressure and optic nerve head biomechanics. Computer methods in biomechanics and biomedical engineering 19, 591–602. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

