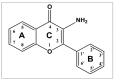
Fluorimetric Properties of 3-Aminoflavone Biomolecule (3-AF). X-ray Crystal Structure of New Polymorph of 3-AF
- PMID: 31412581
- PMCID: PMC6719987
- DOI: 10.3390/molecules24162927
Fluorimetric Properties of 3-Aminoflavone Biomolecule (3-AF). X-ray Crystal Structure of New Polymorph of 3-AF
Abstract
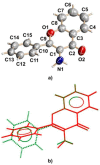
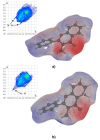
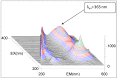
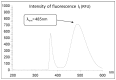
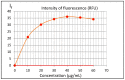
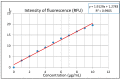
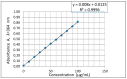
The crystal structure of the new polymorphic form of 3-aminoflavone (3-AF) has been determined by single crystal X-ray diffraction. This report presents results of fluorimetric studies on 3-AF in methanol and aquatic solvents. Based on 3D fluorescence emission spectra, optimal values for excitation (λex) and emission/analytical (λem) wavelength, the analytical concentration range as well as the range of concentration quenching for the studied compound were established. Moreover, the limit of detection (LOD) and the limit of quantification (LOQ) were determined. The results were compared with those obtained using the standard UV-Vis absorption spectrophotometric method. The effect of acidity (pH) and the concentration of halide anions (chlorides, bromides, iodides and fluorides) on fluorescence quenching were analysed.
Keywords: 3-aminoflavone; LOD (limit of detection); LOQ (limit of quantification); X-ray crystallography; concentration quenching; fluorescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kośmider B., Osiecka E. Flavonoid compounds: A review of anticancer properties and interactions with cis-diamminedichloroplatinum(II) Drug Dev. Res. 2004;63:200–211. doi: 10.1002/ddr.10421. - DOI
-
- Nowak A., Zielonka J., Turek M., Klimowicz A. Wpływ przeciwutleniaczy zawartych w owocach na proces fotostarzenia się skóry. Postępy Fitoterapii. 2014;2:94–99.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

