Fluorescence in Situ Hybridization (FISH) for Detecting Anaplastic Lymphoma Kinase (ALK) Rearrangement in Lung Cancer: Clinically Relevant Technical Aspects
- PMID: 31412611
- PMCID: PMC6720438
- DOI: 10.3390/ijms20163939
Fluorescence in Situ Hybridization (FISH) for Detecting Anaplastic Lymphoma Kinase (ALK) Rearrangement in Lung Cancer: Clinically Relevant Technical Aspects
Abstract
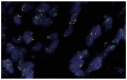
In 2011, the Vysis Break Apart ALK fluorescence in situ hybridization (FISH) assay was approved by the United States Food and Drug Administration as a companion diagnostic for detecting ALK rearrangement in lung cancer patients who may benefit from treatment of tyrosine kinase inhibitor therapy. This assay is the current "gold standard". According to updated ALK testing guidelines from the College of American Pathologists, the International Association for the Study of Lung Cancer and the Association for Molecular Pathology published in 2018, ALK immunohistochemistry is formally an alternative to ALK FISH, and simultaneous detection of multiple hot spots, including, at least, ALK, ROS1, RET, MET, ERBB2, BRAF and KRAS genes is also recommended while performing next generation sequencing (NGS)-based testing. Therefore, ALK status in a specimen can be tested by different methods and platforms, even in the same institution or laboratory. In this review, we discuss several clinically relevant technical aspects of ALK FISH, including pros and cons of the unique two-step (50- to 100-cell) analysis approach employed in the Vysis Break Apart ALK FISH assay, including: the preset cutoff value of ≥15% for a positive result; technical aspects and biology of discordant results obtained by different methods; and incidental findings, such as ALK copy number gain or amplification and co-existent driver mutations. These issues have practical implications for ALK testing in the clinical laboratory following the updated guidelines.
Keywords: ALK; cutoff value; discordant result; fluorescence in situ hybridization (FISH); immunohistochemistry (IHC); incidental finding; next generation sequencing (NGS).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Lung and Brunchus. Volume 2019 American Cancer Society Inc.; Atlanta, GA, USA: 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

