The tumor suppressor FBXO31 preserves genomic integrity by regulating DNA replication and segregation through precise control of cyclin A levels
- PMID: 31413110
- PMCID: PMC6791333
- DOI: 10.1074/jbc.RA118.007055
The tumor suppressor FBXO31 preserves genomic integrity by regulating DNA replication and segregation through precise control of cyclin A levels
Abstract
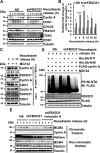
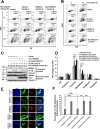
F-box protein 31 (FBXO31) is a reported putative tumor suppressor, and its inactivation due to loss of heterozygosity is associated with cancers of different origins. An emerging body of literature has documented FBXO31's role in preserving genome integrity following DNA damage and in the cell cycle. However, knowledge regarding the role of FBXO31 during normal cell-cycle progression is restricted to its functions during the G2/M phase. Interestingly, FBXO31 levels remain high even during the early G1 phase, a crucial stage for preparing the cells for DNA replication. Therefore, we sought to investigate the functions of FBXO31 during the G1 phase of the cell cycle. Here, using flow cytometric, biochemical, and immunofluorescence techniques, we show that FBXO31 is essential for maintaining optimum expression of the cell-cycle protein cyclin A for efficient cell-cycle progression. Stable FBXO31 knockdown led to atypical accumulation of cyclin A during the G1 phase, driving premature DNA replication and compromised loading of the minichromosome maintenance complex, resulting in replication from fewer origins and DNA double-strand breaks. Because of these inherent defects in replication, FBXO31-knockdown cells were hypersensitive to replication stress-inducing agents and displayed pronounced genomic instability. Upon entering mitosis, the cells defective in DNA replication exhibited a delay in the prometaphase-to-metaphase transition and anaphase defects such as lagging and bridging chromosomes. In conclusion, our findings establish that FBXO31 plays a pivotal role in preserving genomic integrity by maintaining low cyclin A levels during the G1 phase for faithful genome duplication and segregation.
Keywords: DNA replication; F-box protein 31 (FBXO31); LOH; MCM complex; SCF complex; anaphase-promoting complex; cell cycle; genomic instability; protein degradation; tumor suppressor gene.
© 2019 Dutta et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






References
-
- Kumar R., Neilsen P. M., Crawford J., McKirdy R., Lee J., Powell J. A., Saif Z., Martin J. M., Lombaerts M., Cornelisse C. J., Cleton-Jansen A. M., and Callen D. F. (2005) FBXO31 is the chromosome 16q24.3 senescence gene, a candidate breast tumor suppressor, and a component of an SCF complex. Cancer Res. 65, 11304–11313 10.1158/0008-5472.CAN-05-0936 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

