A specific small-molecule inhibitor of protein kinase CδI activity improves metabolic dysfunction in human adipocytes from obese individuals
- PMID: 31413114
- PMCID: PMC6791326
- DOI: 10.1074/jbc.RA119.008777
A specific small-molecule inhibitor of protein kinase CδI activity improves metabolic dysfunction in human adipocytes from obese individuals
Abstract
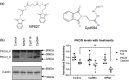
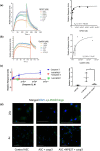
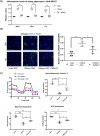
The metabolic consequences and sequelae of obesity promote life-threatening morbidities. PKCδI is an important elicitor of inflammation and apoptosis in adipocytes. Here we report increased PKCδI activation via release of its catalytic domain concurrent with increased expression of proinflammatory cytokines in adipocytes from obese individuals. Using a screening strategy of dual recognition of PKCδI isozymes and a caspase-3 binding site on the PKCδI hinge domain with Schrödinger software and molecular dynamics simulations, we identified NP627, an organic small-molecule inhibitor of PKCδI. Characterization of NP627 by surface plasmon resonance (SPR) revealed that PKCδI and NP627 interact with each other with high affinity and specificity, SPR kinetics revealed that NP627 disrupts caspase-3 binding to PKCδI, and in vitro kinase assays demonstrated that NP627 specifically inhibits PKCδI activity. The SPR results also indicated that NP627 affects macromolecular interactions between protein surfaces. Of note, release of the PKCδI catalytic fragment was sufficient to induce apoptosis and inflammation in adipocytes. NP627 treatment of adipocytes from obese individuals significantly inhibited PKCδI catalytic fragment release, decreased inflammation and apoptosis, and significantly improved mitochondrial metabolism. These results indicate that PKCδI is a robust candidate for targeted interventions to manage obesity-associated chronic inflammatory diseases. We propose that NP627 may also be used in other biological systems to better understand the impact of caspase-3-mediated activation of kinase activity.
Keywords: NP627; PKC; adipocyte; adipose tissue; adipose tissue metabolism; apoptosis; inflammation; metabolic disease; metabolic disorder; obesity.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. This work does not reflect the view or opinion of the James A. Haley VA Hospital or the US Government
Figures




References
-
- Kurokawa J., Nagano H., Ohara O., Kubota N., Kadowaki T., Arai S., and Miyazaki T. (2011) Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc. Natl. Acad. Sci. U.S.A. 108, 12072–12077 10.1073/pnas.1101841108 - DOI - PMC - PubMed
-
- Nisoli E., Briscini L., Giordano A., Tonello C., Wiesbrock S. M., Uysal K. T., Cinti S., Carruba M. O., and Hotamisligil G. S. (2000) Tumor necrosis factor α mediates apoptosis of brown adipocytes and defective brown adipocyte function in obesity. Proc. Natl. Acad. Sci. U.S.A. 97, 8033–8038 10.1073/pnas.97.14.8033 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

