Discovery of Novel Thrips Vector Proteins That Bind to the Viral Attachment Protein of the Plant Bunyavirus Tomato Spotted Wilt Virus
- PMID: 31413126
- PMCID: PMC6803271
- DOI: 10.1128/JVI.00699-19
Discovery of Novel Thrips Vector Proteins That Bind to the Viral Attachment Protein of the Plant Bunyavirus Tomato Spotted Wilt Virus
Abstract
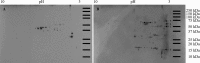
The plant-pathogenic virus tomato spotted wilt virus (TSWV) encodes a structural glycoprotein (GN) that, like with other bunyavirus/vector interactions, serves a role in viral attachment and possibly in entry into arthropod vector host cells. It is well documented that Frankliniella occidentalis is one of nine competent thrips vectors of TSWV transmission to plant hosts. However, the insect molecules that interact with viral proteins, such as GN, during infection and dissemination in thrips vector tissues are unknown. The goals of this project were to identify TSWV-interacting proteins (TIPs) that interact directly with TSWV GN and to localize the expression of these proteins in relation to virus in thrips tissues of principal importance along the route of dissemination. We report here the identification of six TIPs from first-instar larvae (L1), the most acquisition-efficient developmental stage of the thrips vector. Sequence analyses of these TIPs revealed homology to proteins associated with the infection cycle of other vector-borne viruses. Immunolocalization of the TIPs in L1 revealed robust expression in the midgut and salivary glands of F. occidentalis, the tissues most important during virus infection, replication, and plant inoculation. The TIPs and GN interactions were validated using protein-protein interaction assays. Two of the thrips proteins, endocuticle structural glycoprotein and cyclophilin, were found to be consistent interactors with GN These newly discovered thrips protein-GN interactions are important for a better understanding of the transmission mechanism of persistent propagative plant viruses by their vectors, as well as for developing new strategies of insect pest management and virus resistance in plants.IMPORTANCE Thrips-transmitted viruses cause devastating losses to numerous food crops worldwide. For negative-sense RNA viruses that infect plants, the arthropod serves as a host as well by supporting virus replication in specific tissues and organs of the vector. The goal of this work was to identify thrips proteins that bind directly to the viral attachment protein and thus may play a role in the infection cycle in the insect. Using the model plant bunyavirus tomato spotted wilt virus (TSWV), and the most efficient thrips vector, we identified and validated six TSWV-interacting proteins from Frankliniella occidentalis first-instar larvae. Two proteins, an endocuticle structural glycoprotein and cyclophilin, were able to interact directly with the TSWV attachment protein, GN, in insect cells. The TSWV GN-interacting proteins provide new targets for disrupting the viral disease cycle in the arthropod vector and could be putative determinants of vector competence.
Keywords: Bunyavirales; insect vector; orthotospovirus; plant virology; thrips; vector biology; virus-vector interactions.
Copyright © 2019 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

