A randomized controlled trial evaluating the effects of amlodipine on myocardial iron deposition in pediatric patients with thalassemia major
- PMID: 31413542
- PMCID: PMC6659783
- DOI: 10.2147/DDDT.S211630
A randomized controlled trial evaluating the effects of amlodipine on myocardial iron deposition in pediatric patients with thalassemia major
Abstract
Background: Mortality rates increase due to iron deposition in the cardiac muscles of thalassemia major (TM) patients. Iron overload cardiomyopathy could be treated with a combination therapy of an iron chelator and an L-type calcium channel blocker. We designed a randomized controlled study to assess the potential of amlodipine, alongside chelation, in reducing myocardial iron concentration in TM patients compared with a placebo.
Objectives: This study aims to estimate the change in myocardial iron concentration (MIC) determined by magnetic resonance imaging after 6 months of treatment with amlodipine, as well as measuring the changes in the secondary outcomes (liver iron concentration (LIC), serum ferritin level (SF), and left ventricle ejection fraction (LVEF)) of study participants.
Methods: A single, randomized, placebo-controlled trial was performed in 40 β-Thalassemia major patients aged between 6 and 20 years old, who received either oral amlodipine 2.5-5 mg/day or a placebo, in addition to a Deferasirox chelation regimen in a 1:1 allocation ratio.
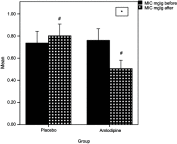
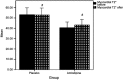
Results: After 6 months, a significant reduction was noted in the MIC of patients receiving amlodipine (n=20), compared with the patients receiving the placebo (n=20). At baseline, the mean was 0.76±0.11 mg/g dry weight, while at 6 months, the mean was 0.51±0.07 mg/g dry weight (p<0.001). Also, there was a significant change in the myocardial T2* after 6 months; the amlodipine increased the myocardial T2* from 40.63±5.45 ms at baseline to 43.25±5.35 ms (p<0.001). However, amlodipine did not significantly affect the secondary outcomes by the end of the study.
Conclusion: The addition of amlodipine to the standard chelation therapy in transfusion-dependent thalassemia major patients improves myocardial iron overload without increasing the adverse effects.
Keywords: amlodipine; magnetic resonance imaging; myocardial iron concentration; thalassemia major.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Tanner MA, Galanello R, Dessi C, et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115(14):1876–1884. doi:10.1161/CIRCULATIONAHA.106.648790 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

