Graphene-based 3D scaffolds in tissue engineering: fabrication, applications, and future scope in liver tissue engineering
- PMID: 31413573
- PMCID: PMC6662516
- DOI: 10.2147/IJN.S192779
Graphene-based 3D scaffolds in tissue engineering: fabrication, applications, and future scope in liver tissue engineering
Abstract
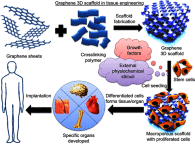
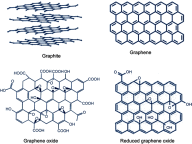
Tissue engineering embraces the potential of recreating and replacing defective body parts by advancements in the medical field. Being a biocompatible nanomaterial with outstanding physical, chemical, optical, and biological properties, graphene-based materials were successfully employed in creating the perfect scaffold for a range of organs, starting from the skin through to the brain. Investigations on 2D and 3D tissue culture scaffolds incorporated with graphene or its derivatives have revealed the capability of this carbon material in mimicking in vivo environment. The porous morphology, great surface area, selective permeability of gases, excellent mechanical strength, good thermal and electrical conductivity, good optical properties, and biodegradability enable graphene materials to be the best component for scaffold engineering. Along with the apt microenvironment, this material was found to be efficient in differentiating stem cells into specific cell types. Furthermore, the scope of graphene nanomaterials in liver tissue engineering as a promising biomaterial is also discussed. This review critically looks into the unlimited potential of graphene-based nanomaterials in future tissue engineering and regenerative therapy.
Keywords: 3D; graphene; liver; microenvironment; regenerative therapy; scaffold; stem cells; tissue engineering.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures








References
-
- Lanza R, Langer R, Vacanti JP. Principles of Tissue Engineering. London: Academic press; 2011.
-
- Minuth WW, Sittinger M, Kloth S. Tissue engineering: generation of differentiated artificial tissues for biomedical applications. Cell Tissue Res. 1997;291(1):1–11. - PubMed
-
- Nerem RM, Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1(1):3–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

