Exposure to the antimicrobial peptide LL-37 produces dendritic cells optimized for immunotherapy
- PMID: 31413918
- PMCID: PMC6682359
- DOI: 10.1080/2162402X.2019.1608106
Exposure to the antimicrobial peptide LL-37 produces dendritic cells optimized for immunotherapy
Abstract
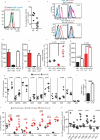
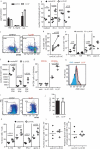
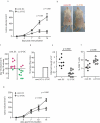
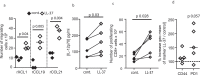
Immunization of patients with autologous, ex vivo matured dendritic cell (DC) preparations, in order to prime antitumor T-cell responses, is the focus of intense research. Despite progress and approval of clinical approaches, significant enhancement of these personalized immunotherapies is urgently needed to improve efficacy. We show that immunotherapeutic murine and human DC, generated in the presence of the antimicrobial host defense peptide LL-37, have dramatically enhanced expansion and differentiation of cells with key features of the critical CD103+/CD141+ DC subsets, including enhanced cross-presentation and co-stimulatory capacity, and upregulation of CCR7 with improved migratory capacity. These LL-37-DC enhanced proliferation, activation and cytokine production by CD8+ (but not CD4+) T cells in vitro and in vivo. Critically, tumor antigen-presenting LL-37-DC increased migration of primed, activated CD8+ T cells into established squamous cell carcinomas in mice, and resulted in tumor regression. This advance therefore has the potential to dramatically enhance DC immunotherapy protocols.
Keywords: CD103; CD141; CD86; CLEC9A; Immunotherapy; PD1; cancer; cathelicidin; cross-presentation; dendritic cells; host defense peptide.
Figures



References
-
- Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EH, Duiveman-de Boer T, van de Rakt MW, Scharenborg NM, de Boer AJ, Pots JM, et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res. 2016;22(9):2155–2166. doi:10.1158/1078-0432.CCR-15-2205. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
