Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: a randomized multicenter Phase IIb trial (TRAVERSE)
- PMID: 31413923
- PMCID: PMC6682346
- DOI: 10.1080/2162402X.2019.1615817
Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: a randomized multicenter Phase IIb trial (TRAVERSE)
Abstract
Pexastimogene devacirepvec (Pexa-Vec) is a vaccinia virus-based oncolytic immunotherapy designed to preferentially replicate in and destroy tumor cells while stimulating anti-tumor immunity by expressing GM-CSF. An earlier randomized Phase IIa trial in predominantly sorafenib-naïve hepatocellular carcinoma (HCC) demonstrated an overall survival (OS) benefit. This randomized, open-label Phase IIb trial investigated whether Pexa-Vec plus Best Supportive Care (BSC) improved OS over BSC alone in HCC patients who failed sorafenib therapy (TRAVERSE). 129 patients were randomly assigned 2:1 to Pexa-Vec plus BSC vs. BSC alone. Pexa-Vec was given as a single intravenous (IV) infusion followed by up to 5 IT injections. The primary endpoint was OS. Secondary endpoints included overall response rate (RR), time to progression (TTP) and safety. A high drop-out rate in the control arm (63%) confounded assessment of response-based endpoints. Median OS (ITT) for Pexa-Vec plus BSC vs. BSC alone was 4.2 and 4.4 months, respectively (HR, 1.19, 95% CI: 0.78-1.80; p = .428). There was no difference between the two treatment arms in RR or TTP. Pexa-Vec was generally well-tolerated. The most frequent Grade 3 included pyrexia (8%) and hypotension (8%). Induction of immune responses to vaccinia antigens and HCC associated antigens were observed. Despite a tolerable safety profile and induction of T cell responses, Pexa-Vec did not improve OS as second-line therapy after sorafenib failure. The true potential of oncolytic viruses may lie in the treatment of patients with earlier disease stages which should be addressed in future studies. ClinicalTrials.gov: NCT01387555.
Keywords: Hepatocellular carcinoma; Pexa-Vec; oncolytic immunotherapy; oncolytic vaccinia; sorafenib.
Figures
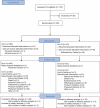
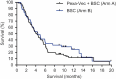
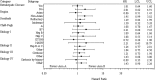
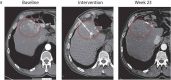

References
-
- GLOBOCAN Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012; 2012. doi:10.1094/PDIS-11-11-0999-PDN - DOI
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
