Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect
- PMID: 31414050
- PMCID: PMC6677551
- DOI: 10.1126/sciadv.aax6946
Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect
Abstract
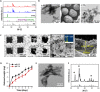
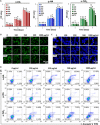
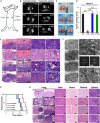
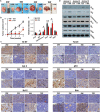
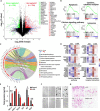
Hydroxyapatite (HA) has been widely applied in bone repair because of its superior biocompatibility. Recently, a proliferation-suppressive effect of HA nanoparticles (n-HA) against various cancer cells was reported. This study was aimed at assessing the translational value of n-HA both as a bone-regenerating material and as an antitumor agent. Inhibition of tumor growth, prevention of metastasis, and enhancement of the survival rate of tumor-bearing rabbits treated with n-HA were demonstrated. Activated mitochondrial-dependent apoptosis in vivo was confirmed, and we observed that a stimulated immune response was involved in the n-HA-induced antitumor effect. A porous titanium scaffold loaded with n-HA was fabricated and implanted into a critical-sized segmental bone defect in a rabbit tumor model. The n-HA-releasing scaffold not only showed a prominent effect in suppressing tumor growth and osteolytic lesion but also promoted bone regeneration. These findings provide a rationale for using n-HA in tumor-associated bone segmental defects.
Figures

References
-
- Randall R. L., A promise to our patients with metastatic bone disease. Ann. Surg. Oncol. 21, 4049–4050 (2014). - PubMed
-
- Andreou D., Bielack S. S., Carrle D., Kevric M., Kotz R., Winkelmann W., Jundt G., Werner M., Fehlberg S., Kager L., Kühne T., Lang S., Dominkus M., Exner G. U., Hardes J., Hillmann A., Ewerbeck V., Heise U., Reichardt P., Tunn P.-U., The influence of tumor- and treatment-related factors on the development of local recurrence in osteosarcoma after adequate surgery. An analysis of 1355 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. Ann. Oncol. 22, 1228–1235 (2011). - PubMed
-
- Ma H., Jiang C., Zhai D., Luo Y., Chen Y., Lv F., Yi Z., Deng Y., Wang J., Chang J., Wu C., A bifunctional biomaterial with photothermal effect for tumor therapy and bone regeneration. Adv. Funct. Mater. 26, 1197–1208 (2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

