NHC-Coordinated Diphosphene-Stabilized Gold(I) Hydride and Its Reversible Conversion to Gold(I) Formate with CO2
- PMID: 31414524
- PMCID: PMC6916326
- DOI: 10.1002/anie.201909798
NHC-Coordinated Diphosphene-Stabilized Gold(I) Hydride and Its Reversible Conversion to Gold(I) Formate with CO2
Abstract
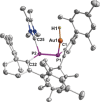
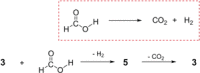
An NHC-coordinated diphosphene is employed as ligand for the synthesis of a hydrocarbon-soluble monomeric AuI hydride, which readily adds CO2 at room temperature yielding the corresponding AuI formate. The reversible reaction can be expedited by the addition of NHC, which induces β-hydride shift and the removal of CO2 from equilibrium through the formation of an NHC-CO2 adduct. The AuI formate is alternatively formed by dehydrogenative coupling of the AuI hydride with formic acid (HCO2 H), thus in total establishing a reaction sequence for the AuI hydride mediated dehydrogenation of HCO2 H as chemical hydrogen storage material.
Keywords: carbon dioxide; diphosphene; gold; ligand design; phosphorus.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



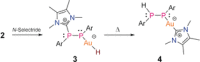




References
-
- None
-
- Crabtree R. H., The Organometallic Chemistry of the Transition Metals, 4th ed., Wiley, Hoboken, 2005;
-
- Hartwig J. F., Organotransition Metal Chemistry From Bonding to Catalysis, University Science Books, Sausalito, 2010;
-
- Corbet J.-P., Mignani G., Chem. Rev. 2006, 106, 2651–2710; - PubMed
-
- van Leeuwen P. W. N. M., Kamer P. C. J., Reek J. N. H., Dierkes P., Chem. Rev. 2000, 100, 2741–2769. - PubMed
LinkOut - more resources
Full Text Sources

