mTOR Inhibitor Therapy Diminishes Circulating CD8+ CD28- Effector Memory T Cells and Improves Allograft Inflammation in Belatacept-refractory Renal Allograft Rejection
- PMID: 31415033
- PMCID: PMC7012662
- DOI: 10.1097/TP.0000000000002917
mTOR Inhibitor Therapy Diminishes Circulating CD8+ CD28- Effector Memory T Cells and Improves Allograft Inflammation in Belatacept-refractory Renal Allograft Rejection
Abstract
Background: Renal allograft rejection is more frequent under belatacept-based, compared with tacrolimus-based, immunosuppression. We studied kidney transplant recipients experiencing rejection under belatacept-based early corticosteroid withdrawal following T-cell-depleting induction in a recent randomized trial (Belatacept-based Early Steroid Withdrawal Trial, clinicaltrials.gov NCT01729494) to determine mechanisms of rejection and treatment.
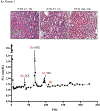
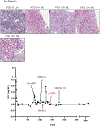
Methods: Peripheral mononuclear cells, serum creatinine levels, and renal biopsies were collected from 8 patients undergoing belatacept-refractory rejection (BRR). We used flow cytometry, histology, and immunofluorescence to characterize CD8 effector memory T cell (TEM) populations in the periphery and graft before and after mammalian target of rapamycin (mTOR) inhibition.
Results: Here, we found that patients with BRR did not respond to standard antirejection therapy and had a substantial increase in alloreactive CD8 T cells with a CD28/DR/CD38/CD45RO TEM. These cells had increased activation of the mTOR pathway, as assessed by phosphorylated ribosomal protein S6 expression. Notably, everolimus (an mTOR inhibitor) treatment of patients with BRR halted the in vivo proliferation of TEM cells and their ex vivo alloreactivity and resulted in their significant reduction in the peripheral blood. The frequency of circulating FoxP3 regulatory T cells was not altered. Importantly, everolimus led to rapid resolution of rejection as confirmed by histology.
Conclusions: Thus, while prior work has shown that concomitant belatacept + mTOR inhibitor therapy is effective for maintenance immunosuppression, our preliminary data suggest that everolimus may provide an available means for effecting "rescue" therapy for rejections occurring under belatacept that are refractory to traditional antirejection therapy with corticosteroids and polyclonal antilymphocyte globulin.
Conflict of interest statement
DISCLOSURE
The authors of this manuscript have no conflict of interest to disclose.
Figures









References
-
- Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(3):443–453. - PubMed
-
- Talawila N, Pengel LH. Does belatacept improve outcomes for kidney transplant recipients? A systematic review. Transplant international : official journal of the European Society for Organ Transplantation. 2015;28(11):1251–1264. - PubMed
-
- Heher E, Markmann JF. The Clearer BENEFITS of Belatacept. The New England journal of medicine. 2016;374(4):388–389. - PubMed
-
- Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(3):535–546. - PubMed
-
- Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374(4):333–343. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

