T cell repertoire remodeling following post-transplant T cell therapy coincides with clinical response
- PMID: 31415240
- PMCID: PMC6819118
- DOI: 10.1172/JCI128323
T cell repertoire remodeling following post-transplant T cell therapy coincides with clinical response
Abstract
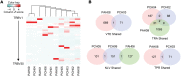
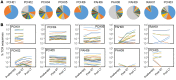
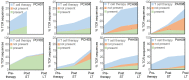
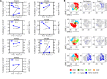
BACKGROUNDImpaired T cell immunity in transplant recipients is associated with infection-related morbidity and mortality. We recently reported the successful use of adoptive T cell therapy (ACT) against drug-resistant/recurrent cytomegalovirus in solid-organ transplant recipients.METHODSIn the present study, we used high-throughput T cell receptor Vβ sequencing and T cell functional profiling to delineate the impact of ACT on T cell repertoire remodeling in the context of pretherapy immunity and ACT products.RESULTSThese analyses indicated that a clinical response was coincident with significant changes in the T cell receptor Vβ landscape after therapy. This restructuring was associated with the emergence of effector memory T cells in responding patients, while nonresponders displayed dramatic pretherapy T cell expansions with minimal change following ACT. Furthermore, immune reconstitution included both adoptively transferred clonotypes and endogenous clonotypes not detected in the ACT products.CONCLUSIONThese observations demonstrate that immune control following ACT requires significant repertoire remodeling, which may be impaired in nonresponders because of the preexisting immune environment. Immunological interventions that can modulate this environment may improve clinical outcomes.TRIAL REGISTRATIONAustralian New Zealand Clinical Trial Registry, ACTRN12613000981729.FUNDINGThis study was supported by funding from the National Health and Medical Research Council, Australia (APP1132519 and APP1062074).
Keywords: Immunology; Immunotherapy; T cells; T-cell receptor; Transplantation.
Conflict of interest statement
Figures
Similar articles
-
Autologous Adoptive T-cell Therapy for Recurrent or Drug-resistant Cytomegalovirus Complications in Solid Organ Transplant Recipients: A Single-arm Open-label Phase I Clinical Trial.Clin Infect Dis. 2019 Feb 1;68(4):632-640. doi: 10.1093/cid/ciy549. Clin Infect Dis. 2019. PMID: 29982441 Clinical Trial.
-
Abundant cytomegalovirus (CMV) reactive clonotypes in the CD8(+) T cell receptor alpha repertoire following allogeneic transplantation.Clin Exp Immunol. 2016 Jun;184(3):389-402. doi: 10.1111/cei.12770. Epub 2016 Mar 8. Clin Exp Immunol. 2016. PMID: 26800118 Free PMC article.
-
Comprehensive Characterization of a Next-Generation Antiviral T-Cell Product and Feasibility for Application in Immunosuppressed Transplant Patients.Front Immunol. 2019 May 28;10:1148. doi: 10.3389/fimmu.2019.01148. eCollection 2019. Front Immunol. 2019. PMID: 31191530 Free PMC article.
-
Refining human T-cell immunotherapy of cytomegalovirus disease: a mouse model with 'humanized' antigen presentation as a new preclinical study tool.Med Microbiol Immunol. 2016 Dec;205(6):549-561. doi: 10.1007/s00430-016-0471-0. Epub 2016 Aug 18. Med Microbiol Immunol. 2016. PMID: 27539576 Review.
-
CMV-specific immunotherapy.Hum Immunol. 2004 May;65(5):558-64. doi: 10.1016/j.humimm.2004.02.003. Hum Immunol. 2004. PMID: 15172457 Review.
Cited by
-
Cytomegalovirus in the transplant setting: Where are we now and what happens next? A report from the International CMV Symposium 2021.Transpl Infect Dis. 2022 Dec;24(6):e13977. doi: 10.1111/tid.13977. Epub 2022 Nov 11. Transpl Infect Dis. 2022. PMID: 36271650 Free PMC article. Review.
-
Complete response to PD-1 blockade following EBV-specific T-cell therapy in metastatic nasopharyngeal carcinoma.NPJ Precis Oncol. 2021 Mar 19;5(1):24. doi: 10.1038/s41698-021-00162-7. NPJ Precis Oncol. 2021. PMID: 33742086 Free PMC article.
-
The Entangled World of Memory T Cells and Implications in Transplantation.Transplantation. 2024 Jan 1;108(1):137-147. doi: 10.1097/TP.0000000000004647. Epub 2023 Dec 13. Transplantation. 2024. PMID: 37271872 Free PMC article. Review.
-
Advances in CAR-Engineered Immune Cell Generation: Engineering Approaches and Sourcing Strategies.Adv Sci (Weinh). 2023 Dec;10(35):e2303215. doi: 10.1002/advs.202303215. Epub 2023 Oct 31. Adv Sci (Weinh). 2023. PMID: 37906032 Free PMC article. Review.
-
Beyond CAR-T: The rise of CAR-NK cell therapy in asthma immunotherapy.J Transl Med. 2024 Aug 5;22(1):736. doi: 10.1186/s12967-024-05534-8. J Transl Med. 2024. PMID: 39103889 Free PMC article. Review.
References
-
- Wang EC, Moss PA, Frodsham P, Lehner PJ, Bell JI, Borysiewicz LK. CD8highCD57+ T lymphocytes in normal, healthy individuals are oligoclonal and respond to human cytomegalovirus. J Immunol. 1995;155(10):5046–5056. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

