Immune adjuvant therapy using Bacillus Calmette-Guérin cell wall skeleton (BCG-CWS) in advanced malignancies: A phase 1 study of safety and immunogenicity assessments
- PMID: 31415377
- PMCID: PMC6831317
- DOI: 10.1097/MD.0000000000016771
Immune adjuvant therapy using Bacillus Calmette-Guérin cell wall skeleton (BCG-CWS) in advanced malignancies: A phase 1 study of safety and immunogenicity assessments
Abstract
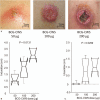
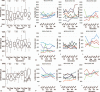
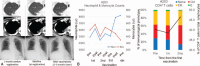
The cell wall skeleton of Bacillus Calmette-Guérin (BCG-CWS) is a bioactive component that is a strong immune adjuvant for cancer immunotherapy. BCG-CWS activates the innate immune system through various pattern recognition receptors and is expected to elicit antigen-specific cellular immune responses when co-administered with tumor antigens. To determine the recommended dose (RD) of BCG-CWS based on its safety profile, we conducted a phase I dose-escalation study of BCG-CWS in combination with WT1 peptide for patients with advanced cancer.The primary endpoint was the proportion of treatment-related adverse events (AEs) at each BCG-CWS dose. The secondary endpoints were immune responses and clinical effects. A BCG-CWS dose of 50, 100, or 200 μg/body was administered intradermally on days 0, 7, 21, and 42, followed by 2 mg of WT1 peptide on the next day. For the escalation of a dose level, 3 + 3 design was used.Study subjects were 18 patients with advanced WT1-expressing cancers refractory to standard anti-cancer therapies (7 melanoma, 5 colorectal, 4 hepatobiliary, 1 ovarian, and 1 lung). Dose-limiting toxicity occurred in the form of local skin reactions in 2 patients at a dose of 200 μg although no serious treatment-related systemic AEs were observed. Neutrophils and monocytes transiently increased in response to BCG-CWS. Some patients demonstrated the induction of the CD4 T cell subset and its differentiation from the naïve to memory phenotype, resulting in a tumor response.The RD of BCG-CWS was determined to be 100 μg/body. This dose was well tolerated and showed promising clinical effects with the induction of an appropriate immune response.
Conflict of interest statement
All authors declared no potential conflicts of interest with regard to this work.
Figures



Similar articles
-
A retrospective study of immunotherapy using the cell wall skeleton of Mycobacterium bovis Bacillus Calmette-Guérin (BCG-CWS) for cervical cancer.Medicine (Baltimore). 2022 Dec 30;101(52):e32481. doi: 10.1097/MD.0000000000032481. Medicine (Baltimore). 2022. PMID: 36595982 Free PMC article.
-
Innate immune therapy with a Bacillus Calmette-Guérin cell wall skeleton after radical surgery for non-small cell lung cancer: a case-control study.Surg Today. 2009;39(3):194-200. doi: 10.1007/s00595-008-3826-3. Epub 2009 Mar 12. Surg Today. 2009. PMID: 19280277
-
Application of BCG-CWS as a Systemic Adjuvant by Using Nanoparticulation Technology.Mol Pharm. 2018 Dec 3;15(12):5762-5771. doi: 10.1021/acs.molpharmaceut.8b00919. Epub 2018 Nov 8. Mol Pharm. 2018. PMID: 30380885
-
Clinical and molecular insights into BCG immunotherapy for melanoma.J Intern Med. 2020 Dec;288(6):625-640. doi: 10.1111/joim.13037. Epub 2020 Mar 4. J Intern Med. 2020. PMID: 32128919 Review.
-
Development of immunoadjuvants for immunotherapy of cancer.Int Immunopharmacol. 2001 Jul;1(7):1249-59. doi: 10.1016/s1567-5769(01)00055-8. Int Immunopharmacol. 2001. PMID: 11460306 Review.
Cited by
-
Trained immunity: A new player in cancer immunotherapy.Elife. 2025 Jun 18;14:e104920. doi: 10.7554/eLife.104920. Elife. 2025. PMID: 40530829 Free PMC article. Review.
-
Advances in Infectious Disease Vaccine Adjuvants.Vaccines (Basel). 2022 Jul 13;10(7):1120. doi: 10.3390/vaccines10071120. Vaccines (Basel). 2022. PMID: 35891284 Free PMC article. Review.
-
Culture filtrate proteins from BCG act as adjuvants for cytotoxic T lymphocyte induction.Front Immunol. 2023 Oct 18;14:1271228. doi: 10.3389/fimmu.2023.1271228. eCollection 2023. Front Immunol. 2023. PMID: 37928526 Free PMC article.
-
Neutrophil Extracellular Traps and Cancer: Trapping Our Attention with Their Involvement in Ovarian Cancer.Int J Mol Sci. 2023 Mar 22;24(6):5995. doi: 10.3390/ijms24065995. Int J Mol Sci. 2023. PMID: 36983067 Free PMC article. Review.
-
Systemic Bacillus Calmette-Guerin Infection One Year After Intravesical Immunotherapy Mimicking Sarcoidosis.Cureus. 2022 Nov 20;14(11):e31697. doi: 10.7759/cureus.31697. eCollection 2022 Nov. Cureus. 2022. PMID: 36561593 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

