Pattern of adverse events induced by aflibercept and ranibizumab: A nationwide spontaneous adverse event reporting database, 2007-2016
- PMID: 31415382
- PMCID: PMC6831246
- DOI: 10.1097/MD.0000000000016785
Pattern of adverse events induced by aflibercept and ranibizumab: A nationwide spontaneous adverse event reporting database, 2007-2016
Abstract
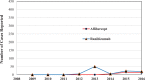
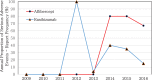
Data regarding the safety of anti-vascular endothelial growth factor (anti-VEGF) treatment is limited.To compare the adverse events (AEs) induced by aflibercept and ranibizumab using a spontaneous reporting system and determine the signals.We used data from the Korea Institute of Drug Safety & Risk Management-Korea Adverse Event Reporting System Database (KIDS-KD), collected between 2007 and 2016. Differences in patient demographics, report type, reporter, causality, and serious-AEs between aflibercept and ranibizumab were compared. Metrics including proportional reporting ratio (PRR), reporting odds ratio (ROR), and information component (IC), were used to compare signals with the AEs on the drug labels in the United States of America and Korea. Logistic regression analysis was performed to identify AEs that are more likely to occur with drug use.A total of 32 aflibercept and 103 ranibizumab cases of AEs were identified. The proportion of AEs that were reported voluntarily was higher with aflibercept (50.5%) use than ranibizumab (4.9%), whereas the AEs reported by post-marketing surveillance were higher with ranibizumab (46.6%) use than aflibercept (31.3%). The percentage of AEs in patients >60 years old, reports by consumers, and the ratio of SAEs to AEs associated with aflibercept (84. %, 9.4%, and 75.0%, respectively) were higher than those of ranibizumab (77.7%, 1.9%, and 19.4%, respectively). The number of newly detected AEs after aflibercept and ranibizumab treatment was 3 and 8, respectively. Among these, conjunctivitis and medicine ineffective were not included on the aflibercept and ranibizumab labels, respectively. Endophthalmitis (OR 6.96, 95% CI 2.74-17.73) was more likely to be reported in patients with aflibercept than in patients without aflibercept, whereas medicine ineffective (OR 18.49, 95% CI 2.39-143.29) and retinal disorder (OR 7.03, 95% CI 1.60-30.96) were more likely to be reported in patients with ranibizumab than in patients without ranibizumab.New signals have been identified for aflibercept and ranibizumab. Further research is necessary to evaluate the causality of AEs that were detected as signals in this study.
Figures
References
-
- Jiang Y, Mieler WF. Update on the use of anti-VEGF Intravitreal therapies for retinal vein occlusions. Asia Pac J Ophthalmol (Phila) 2017;6:546–53. - PubMed
-
- Banaee T, Ashraf M, Conti FF, et al. Switching anti-VEGF drugs in the treatment of diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 2017;48:748–54. - PubMed
-
- Munk MR, Ruckert R, Zinkernagel M, et al. The role of anti-VEGF agents in myopic choroidal neovascularization: Current standards and future outlook. Expert Opin Biol Ther 2016;16:477–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

