Plumbagin Restrains Hepatocellular Carcinoma Angiogenesis by Stromal Cell-Derived Factor (SDF-1)/CXCR4-CXCR7 Axis
- PMID: 31415486
- PMCID: PMC6707097
- DOI: 10.12659/MSM.915782
Plumbagin Restrains Hepatocellular Carcinoma Angiogenesis by Stromal Cell-Derived Factor (SDF-1)/CXCR4-CXCR7 Axis
Abstract
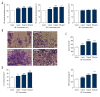
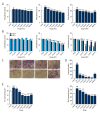
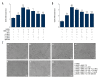
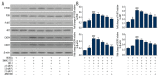
BACKGROUND Anti-angiogenic therapy has recently emerged as a highly promising therapeutic strategy for treating hepatocellular carcinoma (HCC). MATERIAL AND METHODS We assessed cellular proliferation, invasion, and activation of growth factors (VEGF and IL-8) with SDF-1 induced in the hepatocellular carcinoma cell line SMMC-7721, and this progression was limited by plumbagin (PL). The human umbilical vein endothelial cell line HUVEC was co-cultured with SDF-1-induced SMMC-7721, and the expressions of CXCR7, CXCR4, and PI3K/Akt pathways after PL treatment were detected by RT-PCR and Western blot analysis. RESULTS The treatment of the hepatoma cell line SMMC-7721 with SDF-1 resulted in enhanced secretion of the angiogenic factors, IL-8 and VEGF, and shows that these stimulatory effects are abolished by PL. The study further demonstrated that PL not only abolishes SDF-1-induced formation of endothelial tubes, but also inhibits expression of CXCR4 and CXCR7, and partially prevents activation of angiogenic signaling pathways. CONCLUSIONS The effect of PL on the SDF-1-CXCR4/CXCR7 axis has become an attractive target for inhibiting angiogenesis in hepatoma cells. Our results provide more evidence for the clinical application of PL as part of traditional Chinese medicine in modern cancer treatment.
Figures

References
-
- Chen W, Zheng R, Zhang S, et al. Report of cancer incidence and mortality in china, 2010. China Cancer. 2013;40:5. - PubMed
-
- Mercedes F, David S, Jordi B, et al. Angiogenesis in liver disease. J Hepatol. 2009;50:604–20. - PubMed
-
- Wei YF, Jing-Qiang LI, Zhang ZW, et al. [Effects of plumbagin on cell cycle of human hepatic stellate cells stimulated by leptin and its related protein expression]. Chinese Traditional & Herbal Drugs. 2012;43:1776–780. [in Chinese]
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

