Elimination of activating Fcγ receptors in spontaneous autoimmune peripheral polyneuropathy model protects from neuropathic disease
- PMID: 31415574
- PMCID: PMC6695161
- DOI: 10.1371/journal.pone.0220250
Elimination of activating Fcγ receptors in spontaneous autoimmune peripheral polyneuropathy model protects from neuropathic disease
Abstract
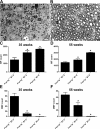
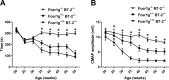
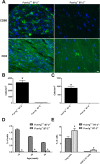
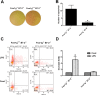
Spontaneous autoimmune peripheral polyneuropathy (SAPP) is a reproducible mouse model of chronic inflammatory peripheral neuropathy in female non-obese diabetic mice deficient in co-stimulatory molecule, B7-2 (also known as CD86). There is evidence that SAPP is an interferon-γ, CD4+ T-cell-mediated disorder, with autoreactive T-cells and autoantibodies directed against myelin protein zero involved in its immunopathogenesis. Precise mechanisms leading to peripheral nerve system inflammation and nerve injury including demyelination in this model are not well defined. We examined the role of activating Fc-gamma receptors (FcγRs) by genetically ablating Fcγ-common chain (Fcer1g) shared by all activating FcγRs in the pathogenesis of this model. We have generated B7-2/ Fcer1g-double null animals for these studies and found that the neuropathic disease is substantially ameliorated in these animals as assessed by behavior, electrophysiology, immunocytochemistry, and morphometry. Our current studies focused on characterizing systemic and endoneurial inflammation in B7-2-null and B7-2/ Fcer1g-double nulls. We found that accumulation of endoneurial inflammatory cells was significantly attenuated in B7-2/ Fcer1g-double nulls compared to B7-2-single nulls. Whereas, systemically the frequency of CD4+ regulatory T cells and expression of immunosuppressive cytokine, IL-10, were significantly enhanced in B7-2/ Fcer1g-double nulls. Overall, these findings suggest that elimination of activating FcγRs modulate nerve injury by altering endoneurial and systemic inflammation. These observations raise the possibility of targeting activating FcγRs as a treatment strategy in acquired inflammatory demyelinating neuropathies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Differential regulation of tissue-resident and blood-derived macrophages in models of autoimmune and traumatic peripheral nerve injury.Front Immunol. 2024 Nov 19;15:1487788. doi: 10.3389/fimmu.2024.1487788. eCollection 2024. Front Immunol. 2024. PMID: 39628475 Free PMC article.
-
Divergent effects of T cell costimulation and inflammatory cytokine production on autoimmune peripheral neuropathy provoked by Aire deficiency.J Immunol. 2013 Apr 15;190(8):3895-904. doi: 10.4049/jimmunol.1203001. Epub 2013 Mar 13. J Immunol. 2013. PMID: 23487421 Free PMC article.
-
Fcγ receptor-mediated inflammation inhibits axon regeneration.PLoS One. 2014 Feb 11;9(2):e88703. doi: 10.1371/journal.pone.0088703. eCollection 2014. PLoS One. 2014. PMID: 24523933 Free PMC article.
-
The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases.Immunobiology. 2012 Nov;217(11):1067-79. doi: 10.1016/j.imbio.2012.07.015. Immunobiology. 2012. PMID: 22964232 Review.
-
Inflammatory neuropathies.Baillieres Clin Neurol. 1994 Apr;3(1):45-72. Baillieres Clin Neurol. 1994. PMID: 7921591 Review.
Cited by
-
Clinical presentation and symptomatology of Guillain-Barré syndrome: A literature review.Medicine (Baltimore). 2024 Jul 26;103(30):e38890. doi: 10.1097/MD.0000000000038890. Medicine (Baltimore). 2024. PMID: 39058828 Free PMC article. Review.
-
Canine leishmaniosis and peripheral neuropathy: a lesson from the neurologist.Parasit Vectors. 2025 Jun 11;18(1):222. doi: 10.1186/s13071-025-06773-4. Parasit Vectors. 2025. PMID: 40500785 Free PMC article.
-
Diagnostic biomarker for type 2 diabetic peripheral neuropathy via comprehensive bioinformatics analysis.J Diabetes. 2024 Mar;16(3):e13506. doi: 10.1111/1753-0407.13506. Epub 2023 Nov 29. J Diabetes. 2024. PMID: 38018513 Free PMC article.
-
The Role of Inflammation in the Pathogenesis of Diabetic Peripheral Neuropathy: New Lessons from Experimental Studies and Clinical Implications.Diabetes Ther. 2025 Mar;16(3):371-411. doi: 10.1007/s13300-025-01699-7. Epub 2025 Feb 10. Diabetes Ther. 2025. PMID: 39928224 Free PMC article. Review.
-
Endoneurial immune interplay in peripheral nerve repair: insights and implications for future therapeutic interventions.Front Neurosci. 2025 May 9;19:1602112. doi: 10.3389/fnins.2025.1602112. eCollection 2025. Front Neurosci. 2025. PMID: 40415889 Free PMC article. Review.
References
-
- Griffin JW, Li CY, Macko C, Ho TW, Hsieh ST, Xue P, et al. Early nodal changes in the acute motor axonal neuropathy pattern of the Guillain-Barre syndrome. J Neurocytol. 1996;25(1):33–51. Epub 1996/01/01. . - PubMed
-
- Prineas JW. Pathology of the Guillain-Barre syndrome. Ann Neurol. 1981;9 Suppl:6–19. Epub 1981/01/01. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

