Preliminary outcomes of preemptive warfarin pharmacogenetic testing at a large rural healthcare center
- PMID: 31415684
- PMCID: PMC6479622
- DOI: 10.1093/ajhp/zxy072
Preliminary outcomes of preemptive warfarin pharmacogenetic testing at a large rural healthcare center
Abstract
Purpose: As a preliminary evaluation of the outcomes of implementing pharmacogenetic testing within a large rural healthcare system, patients who received pre-emptive pharmacogenetic testing and warfarin dosing were monitored until June 2017.
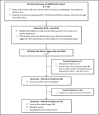
Summary: Over a 20-month period, 749 patients were genotyped for VKORC1 and CYP2C9 as part of the electronic Medical Records and Genomics Pharmacogenetics (eMERGE PGx) study. Of these, 27 were prescribed warfarin and received an alert for pharmacogenetic testing pertinent to warfarin; 20 patients achieved their target international normalized ratio (INR) of 2.0-3.0, and 65% of these patients achieved target dosing within the recommended pharmacogenetic alert dose (± 0.5 mg/day). Of these, 10 patients had never been on warfarin prior to the alert and were further evaluated with regard to time to first stable target INR, bleeds and thromboembolic events, hospitalizations, and mortality. There was a general trend of faster time to first stable target INR when the patient was initiated at a warfarin dose within the alert recommendation versus a dose outside of the alert recommendation with a mean (± SD) of 34 (± 28) days versus 129 (± 117) days, respectively. No trends regarding bleeds, thromboembolic events, hospitalization, or mortality were identified with respect to the pharmacogenetic alert. The pharmacogenetic alert provided pharmacogenetic dosing information to prescribing clinicians and appeared to deploy appropriately with the correct recommendation based upon patient genotype.
Conclusion: Implementing pharmacogenetic testing as a standard of care service in anticoagulation monitoring programs may improve dosage regimens for patients on anticoagulation therapy.
Keywords: CYP2C9; VKORC1; clinical decision support; eMERGE PGx; pharmacogenetics; warfarin.
© American Society of Health-System Pharmacists 2019. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Evaluation of a pharmacogenetic-based warfarin dosing algorithm in patients with low time in therapeutic range - study protocol for a randomized controlled trial.BMC Cardiovasc Disord. 2016 Nov 17;16(1):224. doi: 10.1186/s12872-016-0405-1. BMC Cardiovasc Disord. 2016. PMID: 27855643 Free PMC article. Clinical Trial.
-
Genetic and Non-Genetic Factors Affecting the Quality of Anticoagulation Control and Vascular Events in Atrial Fibrillation.J Stroke Cerebrovasc Dis. 2017 Jun;26(6):1383-1390. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.022. Epub 2017 Apr 12. J Stroke Cerebrovasc Dis. 2017. PMID: 28412319
-
Effect of gene polymorphims on the warfarin treatment at initial stage.Pharmacogenomics J. 2017 Jan;17(1):47-52. doi: 10.1038/tpj.2015.81. Epub 2015 Dec 8. Pharmacogenomics J. 2017. PMID: 26644206
-
Pharmacogenetic differences between warfarin, acenocoumarol and phenprocoumon.Thromb Haemost. 2008 Dec;100(6):1052-7. Thromb Haemost. 2008. PMID: 19132230 Review.
-
Clinical applications of pharmacogenomics guided warfarin dosing.Int J Clin Pharm. 2013 Jun;35(3):359-68. doi: 10.1007/s11096-010-9448-z. Epub 2010 Nov 4. Int J Clin Pharm. 2013. PMID: 21052837 Review.
Cited by
-
The Contribution of Pharmacogenetic Drug Interactions to 90-Day Hospital Readmissions: Preliminary Results from a Real-World Healthcare System.J Pers Med. 2021 Nov 23;11(12):1242. doi: 10.3390/jpm11121242. J Pers Med. 2021. PMID: 34945714 Free PMC article.
-
No Association Between Pharmacogenomics Variants and Hospital and Emergency Department Utilization: A Mayo Clinic Biobank Retrospective Study.Pharmgenomics Pers Med. 2021 Feb 11;14:229-237. doi: 10.2147/PGPM.S281645. eCollection 2021. Pharmgenomics Pers Med. 2021. PMID: 33603442 Free PMC article.
-
Clinical implementation of drug metabolizing gene-based therapeutic interventions worldwide.Hum Genet. 2022 Jun;141(6):1137-1157. doi: 10.1007/s00439-021-02369-x. Epub 2021 Oct 1. Hum Genet. 2022. PMID: 34599365 Review.
-
Pharmacogenetics to guide cardiovascular drug therapy.Nat Rev Cardiol. 2021 Sep;18(9):649-665. doi: 10.1038/s41569-021-00549-w. Epub 2021 May 5. Nat Rev Cardiol. 2021. PMID: 33953382 Free PMC article. Review.
-
Advancing Pharmacogenomics from Single-Gene to Preemptive Testing.Annu Rev Genomics Hum Genet. 2022 Aug 31;23:449-473. doi: 10.1146/annurev-genom-111621-102737. Epub 2022 May 10. Annu Rev Genomics Hum Genet. 2022. PMID: 35537468 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials