Mitochondria in the signaling pathways that control longevity and health span
- PMID: 31415807
- PMCID: PMC7479635
- DOI: 10.1016/j.arr.2019.100940
Mitochondria in the signaling pathways that control longevity and health span
Abstract
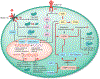
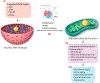
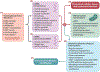
Genetic and pharmacological intervention studies have identified evolutionarily conserved and functionally interconnected networks of cellular energy homeostasis, nutrient-sensing, and genome damage response signaling pathways, as prominent regulators of longevity and health span in various species. Mitochondria are the primary sites of ATP production and are key players in several other important cellular processes. Mitochondrial dysfunction diminishes tissue and organ functional performance and is a commonly considered feature of the aging process. Here we review the evidence that through reciprocal and multilevel functional interactions, mitochondria are implicated in the lifespan modulation function of these pathways, which altogether constitute a highly dynamic and complex system that controls the aging process. An important characteristic of these pathways is their extensive crosstalk and apparent malleability to modification by non-invasive pharmacological, dietary, and lifestyle interventions, with promising effects on lifespan and health span in animal models and potentially also in humans.
Keywords: Aging; DNA repair; Energy; Lifespan; Metabolism; Mitochondria.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest
None.
Figures
References
-
- Aamann MD, Sorensen MM, Hvitby C, Berquist BR, Muftuoglu M, Tian J, de Souza-Pinto NC, Scheibye-Knudsen M, Wilson DM 3rd, Stevnsner T, Bohr VA, 2010. Cockayne syndrome group B protein promotes mitochondrial DNA stability by supporting the DNA repair association with the mitochondrial membrane. FASEB J. 24, 2334–2346. - PMC - PubMed
-
- Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G, 2008. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell. Sci 121, 1046–1053. - PubMed
-
- Akbari M, Krokan HE, 2008. Cytotoxicity and mutagenicity of endogenous DNA base lesions as potential cause of human aging. Mech. Ageing Dev 129, 353–365. - PubMed
-
- Akbari M, Pena-Diaz J, Andersen S, Liabakk NB, Otterlei M, Krokan HE, 2009. Extracts of proliferating and non-proliferating human cells display different base excision pathways and repair fidelity. DNA Repair (Amst.) 8, 834–843. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

