Long-Term Dynamics of Three Dimensional Telomere Profiles in Circulating Tumor Cells in High-Risk Prostate Cancer Patients Undergoing Androgen-Deprivation and Radiation Therapy
- PMID: 31416141
- PMCID: PMC6721586
- DOI: 10.3390/cancers11081165
Long-Term Dynamics of Three Dimensional Telomere Profiles in Circulating Tumor Cells in High-Risk Prostate Cancer Patients Undergoing Androgen-Deprivation and Radiation Therapy
Abstract
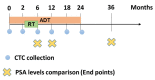
Patient-specific assessment, disease monitoring, and the development of an accurate early surrogate of the therapeutic efficacy of locally advanced prostate cancer still remain a clinical challenge. Contrary to prostate biopsies, circulating tumor cell (CTC) collection from blood is a less-invasive method and has potential as a real-time liquid biopsy and as a surrogate marker for treatment efficacy. In this study, we used size-based filtration to isolate CTCs from the blood of 100 prostate cancer patients with high-risk localized disease. CTCs from five time points: +0, +2, +6, +12 and +24 months were analyzed. Consenting treatment-naïve patients with cT3, Gleason 8-10, or prostate-specific antigen > 20 ng/mL and non-metastatic prostate cancer were included. For all time points, we performed 3D telomere-specific quantitative fluorescence in situ hybridization on a minimum of thirty isolated CTCs. The patients were divided into five groups based on the changes of number of telomeres vs telomere lengths over time and into three clusters based on all telomere parameters found on diagnosis. Group 2 was classified as non-respondent to treatment and the Cluster 3 presented more aggressive phenotype. Additionally, we compared our telomere results with the PSA levels for each patient at 6 months of ADT, at 6 months of completed RT, and at 36 months post-initial therapy. CTCs of patients with PSA levels above or equal to 0.1 ng/mL presented significant increases of nuclear volume, number of telomeres, and telomere aggregates. The 3D telomere analysis of CTCs identified disease heterogeneity among a clinically homogeneous group of patients, which suggests differences in therapeutic responses. Our finding suggests a new opportunity for better treatment monitoring of patients with localized high-risk prostate cancer.
Keywords: androgen deprivation therapy; circulating tumor cells; localized high-risk prostate cancer; radiotherapy; three-dimensional (3D) telomere profiling.
Conflict of interest statement
S.M. is a shareholder, director and chair of the clinical and scientific advisory board of Telo Genomics Corp. (Toronto, ON, Canada). The other authors declare that they have no conflicts of interest.
Figures










References
-
- Nguyen P.L., Martin N.E., Choeurng V., Palmer-Aronsten B., Kolisnik T., Beard C.J., Orio P.F., Nezolosky M.D., Chen Y.W., Shin H., et al. Utilization of biopsy-based genomic classifier to predict distant metastasis after definitive radiation and short-course ADT for intermediate and high-risk prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:186–192. doi: 10.1038/pcan.2016.58. - DOI - PMC - PubMed
-
- Roach M., Hanks G., Thames H., Schellhammer P., Shipley W.U., Sokol G.H., Sandler H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

