Untargeted Assessment of Tumor Fractions in Plasma for Monitoring and Prognostication from Metastatic Breast Cancer Patients Undergoing Systemic Treatment
- PMID: 31416207
- PMCID: PMC6721524
- DOI: 10.3390/cancers11081171
Untargeted Assessment of Tumor Fractions in Plasma for Monitoring and Prognostication from Metastatic Breast Cancer Patients Undergoing Systemic Treatment
Abstract
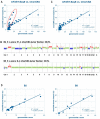
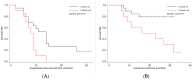
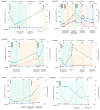
The aim of this study was to assess the prognostic and predictive value of an untargeted assessment of tumor fractions in the plasma of metastatic breast cancer patients and to compare circulating tumor DNA (ctDNA) with circulating tumor cells (CTC) and conventional tumor markers. In metastatic breast cancer patients (n = 29), tumor fractions in plasma were assessed using the untargeted mFAST-SeqS method from 127 serial blood samples. Resulting z-scores for the ctDNA were compared to tumor fractions established with the recently published ichorCNA algorithm and associated with the clinical outcome. We observed a close correlation between mFAST-SeqS z-scores and ichorCNA ctDNA quantifications. Patients with mFAST-SeqS z-scores above three (34.5%) showed significantly worse overall survival (p = 0.014) and progression-free survival (p = 0.018) compared to patients with lower values. Elevated z-score values were clearly associated with radiologically proven progression. The baseline CTC count, carcinoembryonic antigen (CEA), and cancer antigen (CA)15-5 had no prognostic impact on the outcome of patients in the analyzed cohort. This proof of principle study demonstrates the prognostic impact of ctDNA levels detected with mFAST-SeqS as a very fast and cost-effective means to assess the ctDNA fraction without prior knowledge of the genetic landscape of the tumor. Furthermore, mFAST-SeqS-based ctDNA levels provided an early means of measuring treatment response.
Keywords: cell free circulating tumor DNA (ctDNA); circulating tumor cells (CTCs); mFAST-SeqS; metastatic breast cancer (MBC); prognosis; treatment response.
Conflict of interest statement
The authors disclose no potential conflict of interest.
Figures

References
-
- Laessig D., Nagel D., Heinemann V., Untch M., Kahlert S., Bauerfeind I., Stieber P. Importance of cea and ca 15-3 during disease progression in metastatic breast cancer patients. Anticancer Res. 2007;27:1963–1968. - PubMed
-
- Lauro S., Trasatti L., Bordin F., Lanzetta G., Bria E., Gelibter A., Reale M.G., Vecchione A. Comparison of cea, mca, ca 15-3 and ca 27-29 in follow-up and monitoring therapeutic response in breast cancer patients. Anticancer Res. 1999;19:3511–3515. - PubMed
-
- Stieber P., Lässig D., Untch M., Nagel D., Heinemann V. How can cea and ca15-3 be used for estimation of the clinical status and effectiveness of therapy during metastatic breast cancer? J. Clin. Oncol. 2004;22:755. doi: 10.1200/jco.2004.22.14_suppl.755. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

