Multi-Drug/Gene NASH Therapy Delivery and Selective Hyperspectral NIR Imaging Using Chirality-Sorted Single-Walled Carbon Nanotubes
- PMID: 31416250
- PMCID: PMC6721580
- DOI: 10.3390/cancers11081175
Multi-Drug/Gene NASH Therapy Delivery and Selective Hyperspectral NIR Imaging Using Chirality-Sorted Single-Walled Carbon Nanotubes
Abstract
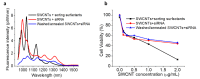
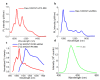
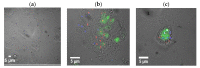
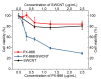
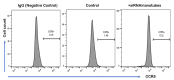
Single-walled carbon nanotubes (SWCNTs) can serve as drug delivery/biological imaging agents, as they exhibit intrinsic fluorescence in the near-infrared, allowing for deeper tissue imaging while providing therapeutic transport. In this work, CoMoCAT (Cobalt Molybdenum Catalyst) SWCNTs, chirality-sorted by aqueous two-phase extraction, are utilized for the first time to deliver a drug/gene combination therapy and image each therapeutic component separately via chirality-specific SWCNT fluorescence. Each of (7,5) and (7,6) sorted SWCNTs were non-covalently loaded with their specific payload: the PI3 kinase inhibitor targeting liver fibrosis or CCR5 siRNA targeting inflammatory pathways with the goal of addressing these processes in nonalcoholic steatohepatitis (NASH), ultimately to prevent its progression to hepatocellular carcinoma. PX-866-(7,5) SWCNTs and siRNA-(7,6) SWCNTs were each imaged via characteristic SWCNT emission at 1024/1120 nm in HepG2 and HeLa cells by hyperspectral fluorescence microscopy. Wavelength-resolved imaging verified the intracellular transport of each SWCNT chirality and drug release. The therapeutic efficacy of each formulation was further demonstrated by the dose-dependent cytotoxicity of SWCNT-bound PX-866 and >90% knockdown of CCR5 expression with SWCNT/siRNA transfection. This study verifies the feasibility of utilizing chirality-sorted SWCNTs for the delivery and component-specific imaging of combination therapies, also suggesting a novel nanotherapeutic approach for addressing the progressions of NASH to hepatocellular carcinoma.
Keywords: NASH; chirality separation; drug-gene delivery; near IR hyperspectral imaging; single-walled carbon nanotubes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Bagalkot V., Zhang L., Levy-Nissenbaum E., Jon S., Kantoff P.W., Langer R., Farokhzad O.C. Quantum Dot−Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Lett. 2007;7:3065–3070. doi: 10.1021/nl071546n. - DOI - PubMed
-
- Guo W., Qiu Z., Guo C., Ding D., Li T., Wang F., Sun J., Zheng N., Liu S. Multifunctional Theranostic Agent of Cu2(OH)PO4 Quantum Dots for Photoacoustic Image-Guided Photothermal/Photodynamic Combination Cancer Therapy. ACS Appl. Mater. Interfaces. 2017;9:9348–9358. doi: 10.1021/acsami.6b15703. - DOI - PubMed
-
- Cheng J., Teply B.A., Sherifi I., Sung J., Luther G., Gu F.X., Levy-Nissenbaum E., Radovic-Moreno A.F., Langer R., Farokhzad O.C. Formulation of functionalized PLGA–PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:869–876. doi: 10.1016/j.biomaterials.2006.09.047. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

