Post-Translational Modifications in NETosis and NETs-Mediated Diseases
- PMID: 31416265
- PMCID: PMC6723044
- DOI: 10.3390/biom9080369
Post-Translational Modifications in NETosis and NETs-Mediated Diseases
Abstract
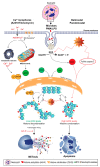
: Neutrophils undergo a unique form of cell death that generates neutrophil extracellular traps (NETs) that may help to neutralize invading pathogens and restore homeostasis. However, uncontrolled NET formation (NETosis) can result in numerous diseases that adversely affect health. Recent studies further elucidate the mechanistic details of the different forms of NETosis and their common end structure, as NETs were constantly found to contain DNA, modified histones and cytotoxic enzymes. In fact, emerging evidence reveal that the post translational modifications (PTMs) of histones in neutrophils have a critical role in regulating neutrophil death. Histone citrullination is shown to promote a rapid form of NET formation independent of NADPH oxidase (NOX), which relies on calcium influx. Interestingly, few studies suggest an association between histone citrullination and other types of PTMs to control cell survival and death, such as histone methylation. Even more exciting is the finding that histone acetylation has a biphasic effect upon NETosis, where histone deacetylase (HDAC) inhibitors promote baseline, NOX-dependent and -independent NETosis. However, increasing levels of histone acetylation suppresses NETosis, and to switch neutrophil death to apoptosis. Interestingly, in the presence of NETosis-promoting stimuli, high levels of HDACis limit both NETosis and apoptosis, and promote neutrophil survival. Recent studies also reveal the importance of the PTMs of neutrophils in influencing numerous pathologies. Histone modifications in NETs can act as a double-edged sword, as they are capable of altering multiple types of neutrophil death, and influencing numerous NET-mediated diseases, such as acute lung injury (ALI), thrombosis, sepsis, systemic lupus erythematosus, and cancer progression. A clear understanding of the role of different PTMs in neutrophils would be important for an understanding of the molecular mechanisms of NETosis, and to appropriately treat NETs-mediated diseases.
Keywords: apoptosis; epigenetics; histone acetylation; histone citrullination; histone deacetylase inhibitors; histone methylation; neutrophil extracellular trap formation; neutrophils; post translational modification.
Conflict of interest statement
The authors declare that they have no conflict of interest related to this manuscript.
Figures
Similar articles
-
Histone Acetylation Promotes Neutrophil Extracellular Trap Formation.Biomolecules. 2019 Jan 18;9(1):32. doi: 10.3390/biom9010032. Biomolecules. 2019. PMID: 30669408 Free PMC article.
-
Histone Deacetylase Inhibitors Dose-Dependently Switch Neutrophil Death from NETosis to Apoptosis.Biomolecules. 2019 May 11;9(5):184. doi: 10.3390/biom9050184. Biomolecules. 2019. PMID: 31083537 Free PMC article.
-
Visualizing NETosis Using a Novel Neutrophil Extracellular Trap-Specific Marker.Methods Mol Biol. 2023;2614:71-80. doi: 10.1007/978-1-0716-2914-7_5. Methods Mol Biol. 2023. PMID: 36587119
-
How Do ROS Induce NETosis? Oxidative DNA Damage, DNA Repair, and Chromatin Decondensation.Biomolecules. 2024 Oct 16;14(10):1307. doi: 10.3390/biom14101307. Biomolecules. 2024. PMID: 39456240 Free PMC article. Review.
-
Cellular Mechanisms of NETosis.Annu Rev Cell Dev Biol. 2020 Oct 6;36:191-218. doi: 10.1146/annurev-cellbio-020520-111016. Epub 2020 Jul 14. Annu Rev Cell Dev Biol. 2020. PMID: 32663035 Free PMC article. Review.
Cited by
-
Human Amniotic Membrane Mesenchymal Stem Cell-Synthesized PGE2 Exerts an Immunomodulatory Effect on Neutrophil Extracellular Trap in a PAD-4-Dependent Pathway through EP2 and EP4.Cells. 2022 Sep 10;11(18):2831. doi: 10.3390/cells11182831. Cells. 2022. PMID: 36139406 Free PMC article.
-
Neutrophils' Extracellular Trap Mechanisms: From Physiology to Pathology.Int J Mol Sci. 2022 Oct 25;23(21):12855. doi: 10.3390/ijms232112855. Int J Mol Sci. 2022. PMID: 36361646 Free PMC article. Review.
-
Liquid biopsy approach to pancreatic cancer.World J Gastrointest Oncol. 2021 Oct 15;13(10):1263-1287. doi: 10.4251/wjgo.v13.i10.1263. World J Gastrointest Oncol. 2021. PMID: 34721766 Free PMC article. Review.
-
Construction of a NETosis-related gene signature for predicting the prognostic status of sepsis patients.Heliyon. 2024 Aug 23;10(17):e36831. doi: 10.1016/j.heliyon.2024.e36831. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39281624 Free PMC article.
-
Conservation of Cell Communication Systems in Invertebrate Host-Defence Mechanisms: Possible Role in Immunity and Disease.Biology (Basel). 2020 Aug 18;9(8):234. doi: 10.3390/biology9080234. Biology (Basel). 2020. PMID: 32824821 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

