New insights into the role of iron in inflammation and atherosclerosis
- PMID: 31416722
- PMCID: PMC6796517
- DOI: 10.1016/j.ebiom.2019.08.014
New insights into the role of iron in inflammation and atherosclerosis
Abstract
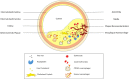
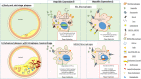
Iron is fundamental for life-essential processes. However, it can also cause oxidative damage, which is thought to trigger numerous pathologies, including cardiovascular diseases. The role of iron in the pathogenesis of atherosclerosis is still not completely understood. Macrophages are both key players in the handling of iron throughout the body and in the onset, progression and destabilization of atherosclerotic plaques. Iron itself might impact atherosclerosis through its effects on macrophages. However, while targeting iron metabolism within macrophages may have some beneficial effects on preventing atherosclerotic plaque progression there may also be negative consequences. Thus, the prevailing view of iron being capable of accelerating the progression of coronary disease through lipid peroxidation may not fully take into account the multi-faceted role of iron in pathogenesis of atherosclerosis. In this review, we will summarize the current understanding of iron metabolism in the context of the complex interplay between iron, inflammation, and atherosclerosis.
Keywords: Atherosclerosis; Hepcidin-ferroportin axis; Intraplaque haemorrhage; Iron metabolism; Macrophages.
Copyright © 2019. Published by Elsevier B.V.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

